Chapter 1 Introduction and Definition of Terms
... A variable which depends only on the state of the system. If a change in the system occurs, the change in such a function depends only on the initial and final state, and is independent of the path of the change. e.g. T, V, P, U, H, S, G. 7. Equation of State A mathematical relationship between the ...
... A variable which depends only on the state of the system. If a change in the system occurs, the change in such a function depends only on the initial and final state, and is independent of the path of the change. e.g. T, V, P, U, H, S, G. 7. Equation of State A mathematical relationship between the ...
ATOM
... – Protons (p), Neutrons (n), and Electrons (e-) • How are they together? • Are they uniformly spread over? ...
... – Protons (p), Neutrons (n), and Electrons (e-) • How are they together? • Are they uniformly spread over? ...
Chemistry Review Fill in the blank
... 7. Exothermic reaction ___________________ energy and energy is shown on the _______________ side. 8. Endothermic reaction __________________ energy and energy is shown on the _______________ side. 9. Independent variable: the variable that you plan to ______________________. 10. Dependent variable: ...
... 7. Exothermic reaction ___________________ energy and energy is shown on the _______________ side. 8. Endothermic reaction __________________ energy and energy is shown on the _______________ side. 9. Independent variable: the variable that you plan to ______________________. 10. Dependent variable: ...
Term paper
... In first case, the electron probability is mostly between the nucleii while in second case, it is outside. So, (+) is more stable. We can extend our calculations to p-orbitals and we find similar wave functions. But, other orbitals have directional character. When, pz has formed σ bond, there is lat ...
... In first case, the electron probability is mostly between the nucleii while in second case, it is outside. So, (+) is more stable. We can extend our calculations to p-orbitals and we find similar wave functions. But, other orbitals have directional character. When, pz has formed σ bond, there is lat ...
Slides from the talk
... cluster's two clouds of hot x-ray emitting gas shown in red. Representing even more mass than the optical galaxies and xray gas combined, the blue hues show the distribution of dark matter in the cluster. Otherwise invisible to telescopic views, the dark matter was mapped by observations of gravitat ...
... cluster's two clouds of hot x-ray emitting gas shown in red. Representing even more mass than the optical galaxies and xray gas combined, the blue hues show the distribution of dark matter in the cluster. Otherwise invisible to telescopic views, the dark matter was mapped by observations of gravitat ...
Lecture I
... area on the properties of the system. • Phase transitions like graphite to diamond conversion, helium normal to superfluid transition, conductor to semiconductor transition, change of boiling point of a liquid with pressure or addition of solute. • Biochemistry-Enzymes and protein stability, DNA sta ...
... area on the properties of the system. • Phase transitions like graphite to diamond conversion, helium normal to superfluid transition, conductor to semiconductor transition, change of boiling point of a liquid with pressure or addition of solute. • Biochemistry-Enzymes and protein stability, DNA sta ...
Review Sheet for Benchmark Exam
... When we did the penny lab, why did we use three pennies even though we only put two in the NaOH solution? What is the third penny called? ...
... When we did the penny lab, why did we use three pennies even though we only put two in the NaOH solution? What is the third penny called? ...
Science 9
... magnesium, calcium, strontium, barium, and radium; all are reactive soft, low density metals. 5. ___________________ are the electrons in the outer shell of an atom, which determine its power to combine with other elements. 6. ___________________ is the regular, repeating pattern in which ions in io ...
... magnesium, calcium, strontium, barium, and radium; all are reactive soft, low density metals. 5. ___________________ are the electrons in the outer shell of an atom, which determine its power to combine with other elements. 6. ___________________ is the regular, repeating pattern in which ions in io ...
Properties of Matter PowerPoint
... produce a change in the composition of matter. Chemical properties can be observed only when the substance in a sample of matter are changing into different substances. ...
... produce a change in the composition of matter. Chemical properties can be observed only when the substance in a sample of matter are changing into different substances. ...
SLIB quantitative chemistry homework
... Consider the following changes imposed upon a sample of gas, assuming the variables not mentioned remain constant: a. What happens to the pressure if the temperature in K is doubled? b. What happens to the volume if the pressure is tripled? c. What happens to the volume if the temperature decreases ...
... Consider the following changes imposed upon a sample of gas, assuming the variables not mentioned remain constant: a. What happens to the pressure if the temperature in K is doubled? b. What happens to the volume if the pressure is tripled? c. What happens to the volume if the temperature decreases ...
Chem Unit 3 Vocabulary
... 16 dynamic condition in which 2 opposing changes occur at equal rates in a closed system 17 pressure exerted by a vapor in equilibrium with its corresponding liquid at a given temperature 18 process by which particles escape from the surface of a non-boiling liquid & enter the gas state 19 substance ...
... 16 dynamic condition in which 2 opposing changes occur at equal rates in a closed system 17 pressure exerted by a vapor in equilibrium with its corresponding liquid at a given temperature 18 process by which particles escape from the surface of a non-boiling liquid & enter the gas state 19 substance ...
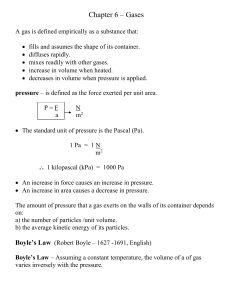
chapter6
... • No ideal gases actually exist. • If they did exist, they would behave exactly as predicted by the gas laws at all temperatures and pressures. • Real gases deviate from the behavior predicted by the gas laws, but under normally encountered temperatures and pressures, the deviations are small. • Con ...
... • No ideal gases actually exist. • If they did exist, they would behave exactly as predicted by the gas laws at all temperatures and pressures. • Real gases deviate from the behavior predicted by the gas laws, but under normally encountered temperatures and pressures, the deviations are small. • Con ...
Chapter 6 notes 2015
... the volume increased by 1/273 of its volume at 0.0oC. The volume decreased with the same ratio for a decrease in temperature. Therefore, theoretically at – 273oC (absolute zero) a gas has no volume. Absolute zero is thought to be the lowest attainable temperature. Charles’ Law – If the pressur ...
... the volume increased by 1/273 of its volume at 0.0oC. The volume decreased with the same ratio for a decrease in temperature. Therefore, theoretically at – 273oC (absolute zero) a gas has no volume. Absolute zero is thought to be the lowest attainable temperature. Charles’ Law – If the pressur ...
111 Exam II Outline
... The Born- Haber cycle uses the law of Hess to determine the Lattice Energy. The lattice energy is the enthalphy change, ∆H, associated when gaseous cations and anions from a crystal: Na+(g) + Cl-(g) NaCl(s) ∆H = - 788KJ Since heat is always evolved in these processes, all lattice energies have a n ...
... The Born- Haber cycle uses the law of Hess to determine the Lattice Energy. The lattice energy is the enthalphy change, ∆H, associated when gaseous cations and anions from a crystal: Na+(g) + Cl-(g) NaCl(s) ∆H = - 788KJ Since heat is always evolved in these processes, all lattice energies have a n ...
Ch 3 Matter & Change
... Each one has a unique name and symbol. In the symbol the first letter is always capitalized and the remaining letter(s) are lowercase. There are 91 naturally occurring elements Who was given credit for organizing them into a table? Dmitri Mendeleev ...
... Each one has a unique name and symbol. In the symbol the first letter is always capitalized and the remaining letter(s) are lowercase. There are 91 naturally occurring elements Who was given credit for organizing them into a table? Dmitri Mendeleev ...
Review - cloudfront.net
... a. More H CO is produced. b. CO concentration increases. c. The equilibrium is pushed in the direction of reactants. d. There is no effect. ____ 66. Which one of the following statements concerning matter is correct? a. A gas has a fixed volume but not a rigid shape. b. A liquid has a fixed volume a ...
... a. More H CO is produced. b. CO concentration increases. c. The equilibrium is pushed in the direction of reactants. d. There is no effect. ____ 66. Which one of the following statements concerning matter is correct? a. A gas has a fixed volume but not a rigid shape. b. A liquid has a fixed volume a ...
Chapter 13…States of Matter
... 4. Miscible: liquids that are capable of dissolving each other 5. Dilute: when a solution has a low concentration of solute 6. Concentrated: when a solution has a high concentration of solute 7. Saturated solution: cannot hold any more of a given solute at a given temperature 8. Unsaturated solution ...
... 4. Miscible: liquids that are capable of dissolving each other 5. Dilute: when a solution has a low concentration of solute 6. Concentrated: when a solution has a high concentration of solute 7. Saturated solution: cannot hold any more of a given solute at a given temperature 8. Unsaturated solution ...
Find your NEW seats Bellringer: Please complete Ms - Parkway C-2
... located near the liquid's edge, escape into the surroundings as vapor (a gas). On the other hand, boiling is a process in which molecules anywhere in the liquid escape, resulting in the formation of vapor bubbles within the liquid. • During evaporation, only those molecules with a certain minimum K. ...
... located near the liquid's edge, escape into the surroundings as vapor (a gas). On the other hand, boiling is a process in which molecules anywhere in the liquid escape, resulting in the formation of vapor bubbles within the liquid. • During evaporation, only those molecules with a certain minimum K. ...
Ay 112 Midterm review
... As pressure increases, the density increases and atoms tend to be more neutral. Also as the density increases lines are broadened by “pressure broadening” or the Stark effect, as the quantum levels beco ...
... As pressure increases, the density increases and atoms tend to be more neutral. Also as the density increases lines are broadened by “pressure broadening” or the Stark effect, as the quantum levels beco ...
Chemical evolution Observation of spiral and irregular galaxies
... No material (gas, stars) enters or leaves the annulus Initially the annulus contains only gas, with no heavy elements (i.e. just hydrogen, helium) As stars are formed, massive stars explode `instantaneously’ as supernovae, returning enriched gas to the ISM Turbulent motions keep the gas well mixed, ...
... No material (gas, stars) enters or leaves the annulus Initially the annulus contains only gas, with no heavy elements (i.e. just hydrogen, helium) As stars are formed, massive stars explode `instantaneously’ as supernovae, returning enriched gas to the ISM Turbulent motions keep the gas well mixed, ...
Document
... Ideal Gas Law and Molecular Formulas: • In high school you used % composition data for compounds to derive corresponding empirical formulas. The Ideal Gas Law Eqtn can be used to determine molar masses. Combining an empirical formula with a molar mass allows a molecular formula to be determined. Em ...
... Ideal Gas Law and Molecular Formulas: • In high school you used % composition data for compounds to derive corresponding empirical formulas. The Ideal Gas Law Eqtn can be used to determine molar masses. Combining an empirical formula with a molar mass allows a molecular formula to be determined. Em ...
The first stars, as seen by supercomputers
... Figure 1. The gathering place. These six panels show density (top; red is less dense; yellow, denser) and temperature (bottom; red is 10 K; yellow, 1000 K) profiles of gas that falls into dark-matter gravitational potentials. (a) Visible here are spoke-like accretion shocks (blue on top; yellow on b ...
... Figure 1. The gathering place. These six panels show density (top; red is less dense; yellow, denser) and temperature (bottom; red is 10 K; yellow, 1000 K) profiles of gas that falls into dark-matter gravitational potentials. (a) Visible here are spoke-like accretion shocks (blue on top; yellow on b ...
HW 10: Electron Configuration Practice -
... Think about the arrangement of electrons and which atom this configuration would represent. In quantum mechanics, the electron configuration is the arrangement of electrons around the nucleus of the atom. An electron configuration provides information about the number of electrons in each orbital. T ...
... Think about the arrangement of electrons and which atom this configuration would represent. In quantum mechanics, the electron configuration is the arrangement of electrons around the nucleus of the atom. An electron configuration provides information about the number of electrons in each orbital. T ...
Study Sheet
... liquid is proportional to the pressure of the gas. Sg = kHPg Qualitatively know how pressure and temperature affect the solubility of gases. (Opening Soda can, freezing water) ...
... liquid is proportional to the pressure of the gas. Sg = kHPg Qualitatively know how pressure and temperature affect the solubility of gases. (Opening Soda can, freezing water) ...