Ethers and Epoxides
... Addition of HO-X to an alkene gives a halohydrin Treatment of a halohydrin with base gives an epoxide Intramolecular Williamson ether synthesis ...
... Addition of HO-X to an alkene gives a halohydrin Treatment of a halohydrin with base gives an epoxide Intramolecular Williamson ether synthesis ...
Chapter 9
... • A 1,2-shift can convert a less stable carbocation into a more stable carbocation. • Rearrangements are not unique to dehydration reactions. • Rearrangements can occur whenever a carbocation is formed as a reactive intermediate. • 2° carbocation A rearranges to the more stable 3° carbocation by a 1 ...
... • A 1,2-shift can convert a less stable carbocation into a more stable carbocation. • Rearrangements are not unique to dehydration reactions. • Rearrangements can occur whenever a carbocation is formed as a reactive intermediate. • 2° carbocation A rearranges to the more stable 3° carbocation by a 1 ...
Chapter 7: Alkene reactions
... It’s a concerted reaction (one step, no intermediate) and pericyclic Cyclic transition state means no rearrangements Product has “Anti-Markovnikov orientation” but…it only APPEARS to violate Markovnikov’s rule…the nucleophile (H-) still ends up on the most substituted C, because this gives the more ...
... It’s a concerted reaction (one step, no intermediate) and pericyclic Cyclic transition state means no rearrangements Product has “Anti-Markovnikov orientation” but…it only APPEARS to violate Markovnikov’s rule…the nucleophile (H-) still ends up on the most substituted C, because this gives the more ...
Chapter 14 Notes
... • The carbonyl group is moderately polar, but it doesn’t have any hydrogen atoms attached, so it cannot hydrogen bond between molecules. ...
... • The carbonyl group is moderately polar, but it doesn’t have any hydrogen atoms attached, so it cannot hydrogen bond between molecules. ...
纳米结构体系物理化学性质的理论研究方法与实例
... and alkynes, the carbonyl C to O double bond in aldehydes and ketones participates in addition reactions and because the C=O bond is polar, it reacts with even more compounds than the nonpolar C to C multiple bonds. • In this section we will discuss the addition of hydrogen, water and alcohols to al ...
... and alkynes, the carbonyl C to O double bond in aldehydes and ketones participates in addition reactions and because the C=O bond is polar, it reacts with even more compounds than the nonpolar C to C multiple bonds. • In this section we will discuss the addition of hydrogen, water and alcohols to al ...
$doc.title
... suffix -‐yl from the root of the carboxylic acid – CH3CO: acetyl; CHO: formyl; C6H5CO: benzoyl ...
... suffix -‐yl from the root of the carboxylic acid – CH3CO: acetyl; CHO: formyl; C6H5CO: benzoyl ...
i m. pharm. - Rajiv Gandhi University of Health Sciences
... sesamum oil cake as the solid substrate. L-glutaminase has received significant attention in recent years owing to its potential applications in medicine as an anticancer agent, as an efficient anti- retroviral agent and as a biosensor. The overall maximum yield of L-glutaminase ( 19.41 U/gds) was a ...
... sesamum oil cake as the solid substrate. L-glutaminase has received significant attention in recent years owing to its potential applications in medicine as an anticancer agent, as an efficient anti- retroviral agent and as a biosensor. The overall maximum yield of L-glutaminase ( 19.41 U/gds) was a ...
Chapter 9. CARBOXYLIC ACIDS AND THEIR DERIVATIVES
... carbon is decreased compared to that of aldehydes and ketones. This means, in general, that carboxylic acids are less reactive towards nucleophilic reagents than carbonyl compounds are. Moreover, a hydroxyl group belongs to poor leaving groups. However, it can be modified or transformed into other f ...
... carbon is decreased compared to that of aldehydes and ketones. This means, in general, that carboxylic acids are less reactive towards nucleophilic reagents than carbonyl compounds are. Moreover, a hydroxyl group belongs to poor leaving groups. However, it can be modified or transformed into other f ...
Synthesis of (−)-Epibatidine - David A. Evans
... reagents did not produce the desired axial alcohol, relatively smaller reducing agents afforded the equatorial alcohol with good selectivity (Table 1). Whereas lithium borohydride was modestly selective for alcohol 16 (86:14; entry 3), the treatment of ketone 15 with NaBH4 in MeOH at -40 °C (entry 4 ...
... reagents did not produce the desired axial alcohol, relatively smaller reducing agents afforded the equatorial alcohol with good selectivity (Table 1). Whereas lithium borohydride was modestly selective for alcohol 16 (86:14; entry 3), the treatment of ketone 15 with NaBH4 in MeOH at -40 °C (entry 4 ...
102 Lecture Ch15
... primary and secondary alcohols, respectively • Also recall that aldehydes are readily oxidized to carboxylic acids, but ketones are not • Tollens’ reagent (silver nitrate plus ammonia) can be used to distinguish between ketones and aldehydes - with aldehydes the Ag2+ is reduced to elemental silver, ...
... primary and secondary alcohols, respectively • Also recall that aldehydes are readily oxidized to carboxylic acids, but ketones are not • Tollens’ reagent (silver nitrate plus ammonia) can be used to distinguish between ketones and aldehydes - with aldehydes the Ag2+ is reduced to elemental silver, ...
Compounds Containing a C=O (Carbonyl) Group
... A secondary (2°) amide contains two C—N bonds. A 2° amide has the structure RCONHR'. A tertiary (3°) amide contains three C—N bonds. A 3° amide ha ...
... A secondary (2°) amide contains two C—N bonds. A 2° amide has the structure RCONHR'. A tertiary (3°) amide contains three C—N bonds. A 3° amide ha ...
Chapter 4. Functional Group Transformations: Oxidation and
... 4.3 Chemoselective agents for oxidizing alcohols MnO2 is a highly chemoselective oxidant-allylic, benzylic, and propargyl alcohols are oxidized faster than saturated alcohols. Solvent: H2O, acetone, or CHCl3. Low reactivity: use large amount of oxidant ...
... 4.3 Chemoselective agents for oxidizing alcohols MnO2 is a highly chemoselective oxidant-allylic, benzylic, and propargyl alcohols are oxidized faster than saturated alcohols. Solvent: H2O, acetone, or CHCl3. Low reactivity: use large amount of oxidant ...
( +)-Limonene Oxidation with Selenium Dioxide
... except carbon-3 (menthol series). Most of these oxidation products are constituents of natural products such as citrus essential oils,2e oi which (+)-limonene is the major constituent. As part of a program to explore conversion of (+)-limonene to more valuable fine chemicals, we studied (+)-limonene ...
... except carbon-3 (menthol series). Most of these oxidation products are constituents of natural products such as citrus essential oils,2e oi which (+)-limonene is the major constituent. As part of a program to explore conversion of (+)-limonene to more valuable fine chemicals, we studied (+)-limonene ...
Staff demonstrating hours for level-3 Inorganic Lab
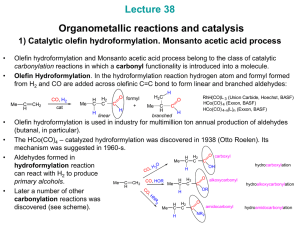
... This is closely related to the nucleophilic displacement reaction in organic chemistry Nu- + RX NuR + XThe nucleophile used are metal centered nucleophiles and are electron rich TM compounds. The most commonly used are metal carbonylate anions. Made with strong reducing agents such as sodium amalg ...
... This is closely related to the nucleophilic displacement reaction in organic chemistry Nu- + RX NuR + XThe nucleophile used are metal centered nucleophiles and are electron rich TM compounds. The most commonly used are metal carbonylate anions. Made with strong reducing agents such as sodium amalg ...
Additional Information on the Synthesis of Esters
... Ethanol is poisonous and its toxicity is increased by the presence of the denaturing substances that are added to laboratory ethanol in order to reduce its illegal consumption. High concentrations of ethanol vapour can be dangerous. Highly flammable. 1-Propanol is harmful to the lungs, skin, eyes an ...
... Ethanol is poisonous and its toxicity is increased by the presence of the denaturing substances that are added to laboratory ethanol in order to reduce its illegal consumption. High concentrations of ethanol vapour can be dangerous. Highly flammable. 1-Propanol is harmful to the lungs, skin, eyes an ...
Alcohols I Reading: Wade chapter 10, sections 10-1- 10
... 1. Organometallic reagents in the synthesis of alcohols Organometallic compounds contain a highly polarized covalent bond between carbon and a metal atom (C–M, M=Li, Na, K, Mg). The C–M bond is polarized so that most of the electron density resides on carbon, since it is the more electronegative ato ...
... 1. Organometallic reagents in the synthesis of alcohols Organometallic compounds contain a highly polarized covalent bond between carbon and a metal atom (C–M, M=Li, Na, K, Mg). The C–M bond is polarized so that most of the electron density resides on carbon, since it is the more electronegative ato ...
Topic 8 specification content - A
... I can explain the nature of intermolecular forces between molecules of polyalkenes, and how its properties can be modified using a plasticizer ...
... I can explain the nature of intermolecular forces between molecules of polyalkenes, and how its properties can be modified using a plasticizer ...
Reactions of Molecules with Oxygen
... Oxidation Reactions of Alcohols What oxidizing agent is used in the selective oxidation of alcohols? Potassium dichromate, K2Cr2O7, is the oxidizing agent [O] used in many alcohol oxidation reactions. The K2Cr2O7 solution must be acidified first by adding sulfuric acid, H2SO4. This provides an appr ...
... Oxidation Reactions of Alcohols What oxidizing agent is used in the selective oxidation of alcohols? Potassium dichromate, K2Cr2O7, is the oxidizing agent [O] used in many alcohol oxidation reactions. The K2Cr2O7 solution must be acidified first by adding sulfuric acid, H2SO4. This provides an appr ...
Chapter 11: Reactions of Alcohols
... Alcohols are more oxidized than alkanes but less oxidized than the corresponding carbonyl compounds such as ketones and aldehydes. The oxidation state or organic molecules can be summarized in the figure on the next slide. In the presence of an oxidizing agent [O], it is possible to change the alcoh ...
... Alcohols are more oxidized than alkanes but less oxidized than the corresponding carbonyl compounds such as ketones and aldehydes. The oxidation state or organic molecules can be summarized in the figure on the next slide. In the presence of an oxidizing agent [O], it is possible to change the alcoh ...
United States Patent
... in the IS-position. Osuka et al .• "Synthesis of Benzochlorin Monomer. Dimer, and Porphyrin-Benzochlorin Heterodimer from 5-Aryl- and 5J5-Diaryloctaethylporphyrins," Bull. Chem. Soc. lpn., 65.3322-30 (1992). Some of these derivatives have shown strong absorptions in the visible region around 700 run ...
... in the IS-position. Osuka et al .• "Synthesis of Benzochlorin Monomer. Dimer, and Porphyrin-Benzochlorin Heterodimer from 5-Aryl- and 5J5-Diaryloctaethylporphyrins," Bull. Chem. Soc. lpn., 65.3322-30 (1992). Some of these derivatives have shown strong absorptions in the visible region around 700 run ...
J. Am. Chem. Soc. 2009, 131, 7962
... ligand are located “under” the WC3H6 ring. A space filling model shows that the three anti protons in the metallacycle are in close contact with isopropyl methyl group protons, making it unlikely that a metallacycle of this type could be formed readily if an anti substituent were present on an R or ...
... ligand are located “under” the WC3H6 ring. A space filling model shows that the three anti protons in the metallacycle are in close contact with isopropyl methyl group protons, making it unlikely that a metallacycle of this type could be formed readily if an anti substituent were present on an R or ...
Slide 1
... decomposition. becomes stronger Bulkier PR3 favor greater linear / branched becomes more hydride-like H aldehyde ratios ( up to 8 : 1). CO Finally, more hydride-like Co-H promotes aldehyde OC Co hydrogenation to alcohols: CO ...
... decomposition. becomes stronger Bulkier PR3 favor greater linear / branched becomes more hydride-like H aldehyde ratios ( up to 8 : 1). CO Finally, more hydride-like Co-H promotes aldehyde OC Co hydrogenation to alcohols: CO ...
CHEM2211_Preps_for_labs_A
... Assess the relationship between carbocation (alkylation of p-xylene and rearrangement and arene nucleophilicity. microscale procedures) e3 Isolate the product. 2. Complete Exercises: pp. e4 Characterize and analyze. 488-493 questions 1, 3, 7, 10 e5 Present, analyze, discuss results in a written repo ...
... Assess the relationship between carbocation (alkylation of p-xylene and rearrangement and arene nucleophilicity. microscale procedures) e3 Isolate the product. 2. Complete Exercises: pp. e4 Characterize and analyze. 488-493 questions 1, 3, 7, 10 e5 Present, analyze, discuss results in a written repo ...
Wolff rearrangement

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.The Wolff rearrangement can be induced via thermolysis, photolysis, or transition metal catalysis. In this last case, the reaction is sensitive to the transition metal; silver (I) oxide or other Ag(I) catalysts work well and are generally used. The Wolff rearrangement has been used in many total syntheses; the most common use is trapping the ketene intermediate with nucleophiles to form carboxylic acid derivatives. The Arndt-Eistert homologation is a specific example of this use, wherein a carboxylic acid may be elongated by a methylene unit. Another common use is in ring-contraction methods; if the α-diazo ketone is cyclic, the Wolff rearrangement results in a ring-contracted product. The Wolff rearrangement works well in generating ring-strained systems, where other reactions may fail.