Isotope Half-Life Radiation Emitted
... http://library.thinkquest.org/06aug/01200/Graphics/705px-Nuclear_fireball.jpg ...
... http://library.thinkquest.org/06aug/01200/Graphics/705px-Nuclear_fireball.jpg ...
1412-PracticeExam4
... protons. B. helium atoms. C. hydrogen atoms. D. helium nuclei. E. electrons. Predict the other product of the following nuclear transformation. A. B. C. D. E. ...
... protons. B. helium atoms. C. hydrogen atoms. D. helium nuclei. E. electrons. Predict the other product of the following nuclear transformation. A. B. C. D. E. ...
Radioisotopes
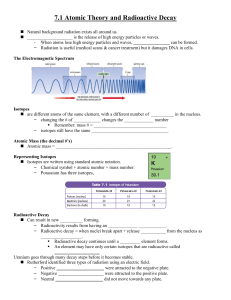
... • Isotopes are any of the different types of atoms (Nuclides) of the same chemical element, each having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have di ...
... • Isotopes are any of the different types of atoms (Nuclides) of the same chemical element, each having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have di ...
AP Chem
... even number of neutrons. The least stable situation is when both numbers are odd. There are only four (or five) stable odd/odd nuclei. Nuclides with a mass number over 200 usually undergo alpha decay. They emit a particle consisting of two protons and two neutrons. Nuclides with too many neutrons un ...
... even number of neutrons. The least stable situation is when both numbers are odd. There are only four (or five) stable odd/odd nuclei. Nuclides with a mass number over 200 usually undergo alpha decay. They emit a particle consisting of two protons and two neutrons. Nuclides with too many neutrons un ...
nuclear chemistry
... Most nuclei are stable. Radionuclides are unstable and spontaneously emit particles and/or electromagnetic radiation U-238 is radioactive o It emits alpha particles(Helium-4 particles) When a nucleus decomposes in this manner, we say it has decayed In nuclear equations, the total number of n ...
... Most nuclei are stable. Radionuclides are unstable and spontaneously emit particles and/or electromagnetic radiation U-238 is radioactive o It emits alpha particles(Helium-4 particles) When a nucleus decomposes in this manner, we say it has decayed In nuclear equations, the total number of n ...
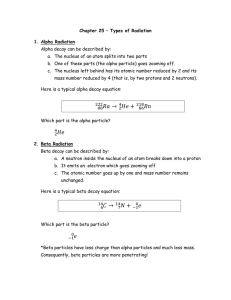
Chapter 25 – Types of Radiation 1. Alpha Radiation Alpha decay
... Positron decay is the mirror image of beta decay and can be described as: a. Something inside the nucleus breaks down causing a proton to become a neutron. b. It emits a positron which goes zooming off. c. The atomic number goes down by one and the mass number remains unchanged. Here is a typical po ...
... Positron decay is the mirror image of beta decay and can be described as: a. Something inside the nucleus breaks down causing a proton to become a neutron. b. It emits a positron which goes zooming off. c. The atomic number goes down by one and the mass number remains unchanged. Here is a typical po ...
Nuclear Decay
... When dysprosium-152 undergoes gamma decay, its nucleus changes from an excited state to a stable state. Write the nuclear reaction equation for this gamma decay. ...
... When dysprosium-152 undergoes gamma decay, its nucleus changes from an excited state to a stable state. Write the nuclear reaction equation for this gamma decay. ...
The discovery of the natural radioactive decay of uranium in 1896 by
... time would be measured in terms of hundreds-to-thousands of millions of years. The next 40 years was a period of expanding research on the nature and behavior of atoms, leading to the development of nuclear fission and fusion as energy sources. A byproduct of this atomic research has been the develo ...
... time would be measured in terms of hundreds-to-thousands of millions of years. The next 40 years was a period of expanding research on the nature and behavior of atoms, leading to the development of nuclear fission and fusion as energy sources. A byproduct of this atomic research has been the develo ...
Atomic Structure and Radioactivity
... Radioactivity was discovered accidentally in 1896. Henri Becquerel found that uranium (U) exposed a photographic plate. In other words, uranium emitted penetrating radiation. ...
... Radioactivity was discovered accidentally in 1896. Henri Becquerel found that uranium (U) exposed a photographic plate. In other words, uranium emitted penetrating radiation. ...
File
... Isotopes are often unstable – they have more neutrons than the element “wants” The isotopes are naturally occurring & decompose at different rates depending on the type of element. A nucleus that is unstable can become stable by undergoing a nuclear reaction (or change) Properties: Alters ph ...
... Isotopes are often unstable – they have more neutrons than the element “wants” The isotopes are naturally occurring & decompose at different rates depending on the type of element. A nucleus that is unstable can become stable by undergoing a nuclear reaction (or change) Properties: Alters ph ...
Nuc Chem PP - Liberty Union High School District
... http://a.abcnews.com/images/Blotter/abc_1radon_ad_070625_ssh.jpg ...
... http://a.abcnews.com/images/Blotter/abc_1radon_ad_070625_ssh.jpg ...
Photo chapter opener 21 Subatomic particle tracks in a bubble
... • A nuclide is a type of atom characterized by its proton number, neutron number and its energy condition. • Nuclides with identical proton number but differing neutron number are called isotopes. • Conditions with a life of less than 10-10s are called excited conditions of a nuclide. • At present, ...
... • A nuclide is a type of atom characterized by its proton number, neutron number and its energy condition. • Nuclides with identical proton number but differing neutron number are called isotopes. • Conditions with a life of less than 10-10s are called excited conditions of a nuclide. • At present, ...
Name
... 5. A self-sustaining reaction in which the products of one reaction event stimulate further reaction events 8. The tendency of some elements, such as uranium, to emit radiation as a result of changes in the atomic nucleus. 9. The conversion of an atomic nucleus of one element into an atomic nucleus ...
... 5. A self-sustaining reaction in which the products of one reaction event stimulate further reaction events 8. The tendency of some elements, such as uranium, to emit radiation as a result of changes in the atomic nucleus. 9. The conversion of an atomic nucleus of one element into an atomic nucleus ...
Ch. 14.2 Notes
... 30. While life processes go on, any carbon -14 nucleus that decays is _____________ by one in the ______________. When the plant or animal dies, the decaying ____________ no longer can be ________________. 31. When archeologists find an ancient item, they can find out how much carbon-14 it has and ...
... 30. While life processes go on, any carbon -14 nucleus that decays is _____________ by one in the ______________. When the plant or animal dies, the decaying ____________ no longer can be ________________. 31. When archeologists find an ancient item, they can find out how much carbon-14 it has and ...
Nuclear Chemistry
... flawed reactor design that was operated with inadequately trained personnel & without proper regard for safety. • The resulting steam explosion & fire released at least five percent of the radioactive reactor core into the atmosphere and downwind. • 28 people died within four months from radiation o ...
... flawed reactor design that was operated with inadequately trained personnel & without proper regard for safety. • The resulting steam explosion & fire released at least five percent of the radioactive reactor core into the atmosphere and downwind. • 28 people died within four months from radiation o ...
Nuclear Reactions
... • differ in their number of neutrons in the nucleus • This gives them a different atomic mass. • The nucleus of an isotope with a certain atomic number and mass is called a nuclide. • Radiation energy is given off from unstable (large) nuclides. – Radioactive decay ...
... • differ in their number of neutrons in the nucleus • This gives them a different atomic mass. • The nucleus of an isotope with a certain atomic number and mass is called a nuclide. • Radiation energy is given off from unstable (large) nuclides. – Radioactive decay ...
Topic Review: Nuclear Chemistry 1. The stability of an isotope
... The rate of decay is called half life. Half-life is a constant that can never be changed. Half life is the measure of the time it takes exactly one half of an amount of isotope to decay. The amount of substance will never decay to zero. Be able to calculate initial amount, fraction remaini ...
... The rate of decay is called half life. Half-life is a constant that can never be changed. Half life is the measure of the time it takes exactly one half of an amount of isotope to decay. The amount of substance will never decay to zero. Be able to calculate initial amount, fraction remaini ...
Document
... Radioactive Decay Can result in new __________ forming. – Radioactivity results from having an _____________ ____________. – Radioactive decay = when nuclei break apart + release ___________ from the nucleus as __________________. Radioactive decay continues until a _________ element forms. An ...
... Radioactive Decay Can result in new __________ forming. – Radioactivity results from having an _____________ ____________. – Radioactive decay = when nuclei break apart + release ___________ from the nucleus as __________________. Radioactive decay continues until a _________ element forms. An ...
Pre-Knowledge: Chemistry and Physics Vocabulary Atomic Number
... isotope is predictable. That is, we cannot predict when a specific atom will decay, but after one half-life half of the atoms will have decayed into a daughter nuclide. ...
... isotope is predictable. That is, we cannot predict when a specific atom will decay, but after one half-life half of the atoms will have decayed into a daughter nuclide. ...
Nuclear - PEO Scarborough Chapter
... 1. Positive - release of positively charged particle called positron and neutrino 2. Negative - release of negatively charged particle called electron and antineutrino. For example, carbon-14 (an unstable isotope of carbon), when subjected to beta decay, forms nitrogen (stable) along with an electro ...
... 1. Positive - release of positively charged particle called positron and neutrino 2. Negative - release of negatively charged particle called electron and antineutrino. For example, carbon-14 (an unstable isotope of carbon), when subjected to beta decay, forms nitrogen (stable) along with an electro ...
Chapter 21 Powerpoint: Nuclear Chemistry
... Nuclear reactions involve the nucleus (protons and neutrons) ...
... Nuclear reactions involve the nucleus (protons and neutrons) ...
Radioactive decay
Radioactive decay, also known as nuclear decay or radioactivity, is the process by which a nucleus of an unstable atom loses energy by emitting radiation. A material that spontaneously emits such radiation — which includes alpha particles, beta particles, gamma rays and conversion electrons — is considered radioactive.Radioactive decay is a stochastic (i.e. random) process at the level of single atoms, in that, according to quantum theory, it is impossible to predict when a particular atom will decay. The chance that a given atom will decay never changes, that is, it does not matter how long the atom has existed. For a large collection of atoms however, the decay rate for that collection can be calculated from their measured decay constants or half-lives. This is the basis of radiometric dating. The half-lives of radioactive atoms have no known limits for shortness or length of duration, and range over 55 orders of magnitude in time.There are many types of radioactive decay (see table below). A decay, or loss of energy from the nucleus, results when an atom with one type of nucleus, called the parent radionuclide (or parent radioisotope), transforms into an atom with a nucleus in a different state, or with a nucleus containing a different number of protons and neutrons. The product is called the daughter nuclide. In some decays, the parent and the daughter nuclides are different chemical elements, and thus the decay process results in the creation of an atom of a different element. This is known as a nuclear transmutation.The first decay processes to be discovered were alpha decay, beta decay, and gamma decay. Alpha decay occurs when the nucleus ejects an alpha particle (helium nucleus). This is the most common process of emitting nucleons, but in rarer types of decays, nuclei can eject protons, or in the case of cluster decay specific nuclei of other elements. Beta decay occurs when the nucleus emits an electron or positron and a neutrino, in a process that changes a proton to a neutron or the other way about. The nucleus may capture an orbiting electron, causing a proton to convert into a neutron in a process called electron capture. All of these processes result in a well-defined nuclear transmutation.By contrast, there are radioactive decay processes that do not result in a nuclear transmutation. The energy of an excited nucleus may be emitted as a gamma ray in a process called gamma decay, or be used to eject an orbital electron by its interaction with the excited nucleus, in a process called internal conversion. Highly excited neutron-rich nuclei, formed as the product of other types of decay, occasionally lose energy by way of neutron emission, resulting in a change of an element from one isotope to another. Another type of radioactive decay results in products that are not defined, but appear in a range of ""pieces"" of the original nucleus. This decay, called spontaneous fission, happens when a large unstable nucleus spontaneously splits into two (and occasionally three) smaller daughter nuclei, and generally leads to the emission of gamma rays, neutrons, or other particles from those products.For a summary table showing the number of stable and radioactive nuclides in each category, see radionuclide. There exist twenty-nine chemical elements on Earth that are radioactive. They are those that contain thirty-four radionuclides that date before the time of formation of the solar system, and are known as primordial nuclides. Well-known examples are uranium and thorium, but also included are naturally occurring long-lived radioisotopes such as potassium-40. Another fifty or so shorter-lived radionuclides, such as radium and radon, found on Earth, are the products of decay chains that began with the primordial nuclides, and ongoing cosmogenic processes, such as the production of carbon-14 from nitrogen-14 by cosmic rays. Radionuclides may also be produced artificially in particle accelerators or nuclear reactors, resulting in 650 of these with half-lives of over an hour, and several thousand more with even shorter half-lives. See this list of nuclides for a list of these, sorted by half life.