Santee Education Complex Chemistry Mini Assessment 11
... a. 1H2 + 1H2 → 2He4 b. 0n1 + 13Al27 → 11Na24 + 2He4 c. 13Al27 + 2He4 → 15P30 +0n1 d. 7N14 + 2He4 →1H1 + 8O17 14) A process in which a very heavy nucleus splits into more stable nuclei of intermediate mass is called: a. nuclear fission. b. a chain reaction. c. nuclear fusion. d. radiocarbon dating. 1 ...
... a. 1H2 + 1H2 → 2He4 b. 0n1 + 13Al27 → 11Na24 + 2He4 c. 13Al27 + 2He4 → 15P30 +0n1 d. 7N14 + 2He4 →1H1 + 8O17 14) A process in which a very heavy nucleus splits into more stable nuclei of intermediate mass is called: a. nuclear fission. b. a chain reaction. c. nuclear fusion. d. radiocarbon dating. 1 ...
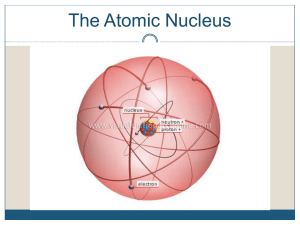
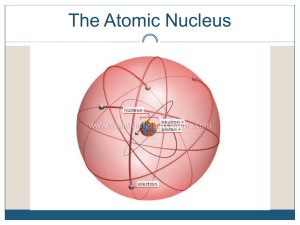
Nuclear Chemistry
... Consists of 2 protons, 2 neutrons emitted during decay Helium nucleus ( 24He )—how particle represented Can be stopped by paper, low energy Atomic number goes down 2, atomic mass goes down 4. Equation: 84210 Po 82206 Pb + 24 He ...
... Consists of 2 protons, 2 neutrons emitted during decay Helium nucleus ( 24He )—how particle represented Can be stopped by paper, low energy Atomic number goes down 2, atomic mass goes down 4. Equation: 84210 Po 82206 Pb + 24 He ...
Measurement of the half-life of
... the AVF cyclotron at Research Center for Nuclear Physics, Osaka University. The cyclotron was operated at an α-particle energy of 40 MeV. The Ti target was placed in close contact with an aluminium degrader to adjust the alpha-particle energy to 30 MeV in the middle of target. After the 30 min irrad ...
... the AVF cyclotron at Research Center for Nuclear Physics, Osaka University. The cyclotron was operated at an α-particle energy of 40 MeV. The Ti target was placed in close contact with an aluminium degrader to adjust the alpha-particle energy to 30 MeV in the middle of target. After the 30 min irrad ...
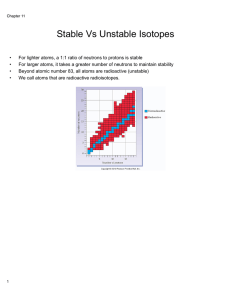
Nuclear Stability
... • All nuclides with 84 or more protons are unstable with respect to radio active decay. • Light nuclides are stable when neutron/proton = 1. For heavier elements the neutron /proton ratio required for stability is greater than 1 and increases with Z. • Nuclides with even numbers of protons and neutr ...
... • All nuclides with 84 or more protons are unstable with respect to radio active decay. • Light nuclides are stable when neutron/proton = 1. For heavier elements the neutron /proton ratio required for stability is greater than 1 and increases with Z. • Nuclides with even numbers of protons and neutr ...
Multiple Choice Questions
... 3. Which nuclear emission is negatively charged? (1) an alpha particle (2) a beta particle (3) a neutron (4) an positron 4. Which nuclear emission has the greatest mass and the least penetrating power? (1) an alpha particle (2) a beta particle (3) a neutron (4) gamma rays 5. Which equation represent ...
... 3. Which nuclear emission is negatively charged? (1) an alpha particle (2) a beta particle (3) a neutron (4) an positron 4. Which nuclear emission has the greatest mass and the least penetrating power? (1) an alpha particle (2) a beta particle (3) a neutron (4) gamma rays 5. Which equation represent ...
File - Dr. Wall`s Science
... • Put experiment in desk drawer, where there was no light • Image still showed up on paper, due to energy from minerals • This energy is nuclear radiation ...
... • Put experiment in desk drawer, where there was no light • Image still showed up on paper, due to energy from minerals • This energy is nuclear radiation ...
Chapter 2, section 4 Formation of Elements
... of the man-made isotopes are unstable. Unstable isotopes can become stable by releasing different types of particles. This process is called radioactive decay and the elements which undergo this process are called radioisotopes/. ...
... of the man-made isotopes are unstable. Unstable isotopes can become stable by releasing different types of particles. This process is called radioactive decay and the elements which undergo this process are called radioisotopes/. ...
Concept Lecture Outline – Radioactivity and Nuclear Reactions
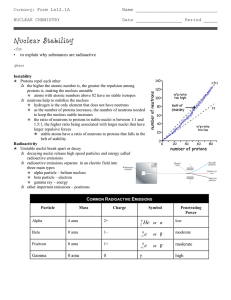
... 1. Radioactive elements a. All elements beyond #83 are radioactive, but isotopes of many others are, also. b. The nuclei of radioactive elements are unstable. c. Radioactivity is the emission of high energy radiation or particles from the nucleus of an unstable atom. 2. A nuclide is the nucleus of a ...
... 1. Radioactive elements a. All elements beyond #83 are radioactive, but isotopes of many others are, also. b. The nuclei of radioactive elements are unstable. c. Radioactivity is the emission of high energy radiation or particles from the nucleus of an unstable atom. 2. A nuclide is the nucleus of a ...
Chapter 19 Radioactive Material An Isotope is an element with a
... 5. Electron Capture: when one of the inner orbital electrons is pulled into the nucleus. Causes no change in mass number but a decrease in the atomic number. Does not happen very often. Particle -‐1 ...
... 5. Electron Capture: when one of the inner orbital electrons is pulled into the nucleus. Causes no change in mass number but a decrease in the atomic number. Does not happen very often. Particle -‐1 ...
Nuclear Stability
... protons is, making the nucleus unstable p atoms with atomic numbers above 82 have no stable isotopes q neutrons help to stabilize the nucleus p hydrogen is the only element that does not have neutrons p as the number of protons increases, the number of neutrons needed to keep the nucleus stable incr ...
... protons is, making the nucleus unstable p atoms with atomic numbers above 82 have no stable isotopes q neutrons help to stabilize the nucleus p hydrogen is the only element that does not have neutrons p as the number of protons increases, the number of neutrons needed to keep the nucleus stable incr ...
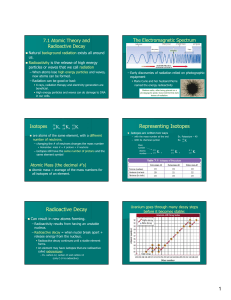
The Band of Stability
... Radioactivity is the spontaneous emission of radiation by nuclei. Radioactive decay changes the nature and identity of an atom’s nucleus. This occurs for a specific reason. Elements from hydrogen to lead (atomic numbers 1-82) have stable isotopes in which the tendency of protons to repel one another ...
... Radioactivity is the spontaneous emission of radiation by nuclei. Radioactive decay changes the nature and identity of an atom’s nucleus. This occurs for a specific reason. Elements from hydrogen to lead (atomic numbers 1-82) have stable isotopes in which the tendency of protons to repel one another ...
Nuclear Decay
... • Step 1. A nucleus releases an alpha particle (2 neutrons and 2 protons) decreasing the mass number of the nucleus • What does that mean? – The original atom is no longer the same – The atomic number is decreased by 2 ...
... • Step 1. A nucleus releases an alpha particle (2 neutrons and 2 protons) decreasing the mass number of the nucleus • What does that mean? – The original atom is no longer the same – The atomic number is decreased by 2 ...
7.2 - Haiku
... • Then Marie and Pierre Curie discovered more radioactive elements including polonium and radium. • Scientists soon realised that there were three different types of radiation. • These were called alpha (α), beta (β), and gamma (γ) rays • from the first three letters of the Greek alphabet. ...
... • Then Marie and Pierre Curie discovered more radioactive elements including polonium and radium. • Scientists soon realised that there were three different types of radiation. • These were called alpha (α), beta (β), and gamma (γ) rays • from the first three letters of the Greek alphabet. ...
NUCLEAR CHEMISTRY
... a. Travel at speeds close to the speed of light b. Penetrating ability about 100 times greater than that of alpha particles. c. They have a range of a few meters in air. 3. Gamma rays a. Greatest penetrating ability b. Protection requires shielding with thick layers of lead, cement, or both ...
... a. Travel at speeds close to the speed of light b. Penetrating ability about 100 times greater than that of alpha particles. c. They have a range of a few meters in air. 3. Gamma rays a. Greatest penetrating ability b. Protection requires shielding with thick layers of lead, cement, or both ...
1 AP Chemistry Chapter 21 - The Nucleus: A Chemist`s View 21.1
... a. Inner orbital electron is captured by the nucleus of its own atom b. Electron combines with a proton and a neutron is formed ...
... a. Inner orbital electron is captured by the nucleus of its own atom b. Electron combines with a proton and a neutron is formed ...
Chapter 18 Notes
... a. Inner orbital electron is captured by the nucleus of its own atom b. Electron combines with a proton and a neutron is formed ...
... a. Inner orbital electron is captured by the nucleus of its own atom b. Electron combines with a proton and a neutron is formed ...
Chapter 19 Nuclear Chemistry
... • Nucleus undergoes decomposition (or decay) to form a different nucleus. ...
... • Nucleus undergoes decomposition (or decay) to form a different nucleus. ...
CH2ch19_1
... 2) Bombarding Nuclides with other nuclides or particles can lead to new Nuclides 3) Most of the “trans-Uranium” elements were synthesized this way (Z = 93-112) a) Neutron Bombardment ...
... 2) Bombarding Nuclides with other nuclides or particles can lead to new Nuclides 3) Most of the “trans-Uranium” elements were synthesized this way (Z = 93-112) a) Neutron Bombardment ...
Representing Isotopes Radioactive Decay
... release energy from the nucleus. Radioactive decay continues until a stable element forms. An element may have isotopes that are radioactive called radioisotopes – Ex. carbon-12, carbon-13 and carbon-14 (only C-14 is radioactive) ...
... release energy from the nucleus. Radioactive decay continues until a stable element forms. An element may have isotopes that are radioactive called radioisotopes – Ex. carbon-12, carbon-13 and carbon-14 (only C-14 is radioactive) ...
Unit 2: The Atom
... •Alpha decay is how elements greater than Bismuth try to become stable. •They will emit an alpha particle (2 neutrons and 2 protons) to try to become stable. •Alpha reactions will always have He on the right side! •To balance: write the upper and lower equations! ...
... •Alpha decay is how elements greater than Bismuth try to become stable. •They will emit an alpha particle (2 neutrons and 2 protons) to try to become stable. •Alpha reactions will always have He on the right side! •To balance: write the upper and lower equations! ...
radioactivity-ppt
... unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. There are numerous types of radioactive decay. The ...
... unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. There are numerous types of radioactive decay. The ...
Radioactivity
... unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. There are numerous types of radioactive decay. The ...
... unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. There are numerous types of radioactive decay. The ...
Radioactive decay
Radioactive decay, also known as nuclear decay or radioactivity, is the process by which a nucleus of an unstable atom loses energy by emitting radiation. A material that spontaneously emits such radiation — which includes alpha particles, beta particles, gamma rays and conversion electrons — is considered radioactive.Radioactive decay is a stochastic (i.e. random) process at the level of single atoms, in that, according to quantum theory, it is impossible to predict when a particular atom will decay. The chance that a given atom will decay never changes, that is, it does not matter how long the atom has existed. For a large collection of atoms however, the decay rate for that collection can be calculated from their measured decay constants or half-lives. This is the basis of radiometric dating. The half-lives of radioactive atoms have no known limits for shortness or length of duration, and range over 55 orders of magnitude in time.There are many types of radioactive decay (see table below). A decay, or loss of energy from the nucleus, results when an atom with one type of nucleus, called the parent radionuclide (or parent radioisotope), transforms into an atom with a nucleus in a different state, or with a nucleus containing a different number of protons and neutrons. The product is called the daughter nuclide. In some decays, the parent and the daughter nuclides are different chemical elements, and thus the decay process results in the creation of an atom of a different element. This is known as a nuclear transmutation.The first decay processes to be discovered were alpha decay, beta decay, and gamma decay. Alpha decay occurs when the nucleus ejects an alpha particle (helium nucleus). This is the most common process of emitting nucleons, but in rarer types of decays, nuclei can eject protons, or in the case of cluster decay specific nuclei of other elements. Beta decay occurs when the nucleus emits an electron or positron and a neutrino, in a process that changes a proton to a neutron or the other way about. The nucleus may capture an orbiting electron, causing a proton to convert into a neutron in a process called electron capture. All of these processes result in a well-defined nuclear transmutation.By contrast, there are radioactive decay processes that do not result in a nuclear transmutation. The energy of an excited nucleus may be emitted as a gamma ray in a process called gamma decay, or be used to eject an orbital electron by its interaction with the excited nucleus, in a process called internal conversion. Highly excited neutron-rich nuclei, formed as the product of other types of decay, occasionally lose energy by way of neutron emission, resulting in a change of an element from one isotope to another. Another type of radioactive decay results in products that are not defined, but appear in a range of ""pieces"" of the original nucleus. This decay, called spontaneous fission, happens when a large unstable nucleus spontaneously splits into two (and occasionally three) smaller daughter nuclei, and generally leads to the emission of gamma rays, neutrons, or other particles from those products.For a summary table showing the number of stable and radioactive nuclides in each category, see radionuclide. There exist twenty-nine chemical elements on Earth that are radioactive. They are those that contain thirty-four radionuclides that date before the time of formation of the solar system, and are known as primordial nuclides. Well-known examples are uranium and thorium, but also included are naturally occurring long-lived radioisotopes such as potassium-40. Another fifty or so shorter-lived radionuclides, such as radium and radon, found on Earth, are the products of decay chains that began with the primordial nuclides, and ongoing cosmogenic processes, such as the production of carbon-14 from nitrogen-14 by cosmic rays. Radionuclides may also be produced artificially in particle accelerators or nuclear reactors, resulting in 650 of these with half-lives of over an hour, and several thousand more with even shorter half-lives. See this list of nuclides for a list of these, sorted by half life.