PreAP Chemistry Radioactivity WS Name Period ____ Match the
... 18. Explain why the gamma rays do not bend. ...
... 18. Explain why the gamma rays do not bend. ...
Unit 14 Notes - shscience.net
... During positron emission, a proton changes to a neutron and positron (which is emitted) Atomic # , mass # doesn’t change ...
... During positron emission, a proton changes to a neutron and positron (which is emitted) Atomic # , mass # doesn’t change ...
Types of Radiation
... Students know the energy release per gram of material is much larger in nuclear fusion or fission reactions than in chemical reactions. The change in mass (calculated by E = mc 2) is small but significant in nuclear reactions. (11b) Students know some naturally occurring isotopes of elements are ...
... Students know the energy release per gram of material is much larger in nuclear fusion or fission reactions than in chemical reactions. The change in mass (calculated by E = mc 2) is small but significant in nuclear reactions. (11b) Students know some naturally occurring isotopes of elements are ...
Unit 3 – Atomic Structure
... • Mass number -The total number of protons and neutrons in a nucleus • Subatomic particles -The three kinds of particles that make up atoms: protons, neutrons, and electrons. • Nuclear fission - Splitting of the nucleus into smaller nuclei • Nuclear fusion - Combining nuclei of light elements into a ...
... • Mass number -The total number of protons and neutrons in a nucleus • Subatomic particles -The three kinds of particles that make up atoms: protons, neutrons, and electrons. • Nuclear fission - Splitting of the nucleus into smaller nuclei • Nuclear fusion - Combining nuclei of light elements into a ...
nuclear chemistry notes 1
... Large atoms with around 83 protons or more are so large that they too are unstable. If atoms are too unstable, they will decompose, or radioactively decay, releasing different types of particles in order to become more stable. ...
... Large atoms with around 83 protons or more are so large that they too are unstable. If atoms are too unstable, they will decompose, or radioactively decay, releasing different types of particles in order to become more stable. ...
document
... emissions. Each alpha emission is shown as a diagonal to the left and each beta emission is a horizontal line to the right. ...
... emissions. Each alpha emission is shown as a diagonal to the left and each beta emission is a horizontal line to the right. ...
Nuclear Power Plant Notes
... • Circle each atom at right that would be an isotope of the atom below. Look on the period table, what element does the atom below represent? ...
... • Circle each atom at right that would be an isotope of the atom below. Look on the period table, what element does the atom below represent? ...
5.7 Nuclear Radiation
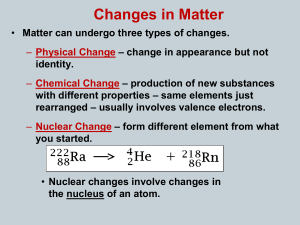
... • Matter can undergo three types of changes. – Physical Change – change in appearance but not identity. – Chemical Change – production of new substances with different properties – same elements just rearranged – usually involves valence electrons. – Nuclear Change – form different element from what ...
... • Matter can undergo three types of changes. – Physical Change – change in appearance but not identity. – Chemical Change – production of new substances with different properties – same elements just rearranged – usually involves valence electrons. – Nuclear Change – form different element from what ...
Radioactivity - Teach Nuclear
... Atomic number increases by 1 (new element) During this conversion an electron and an antineutrino are ejected from the nucleus ...
... Atomic number increases by 1 (new element) During this conversion an electron and an antineutrino are ejected from the nucleus ...
Radiation and Radioactive Decay
... Why is it that the middle elements have the highest binding energies? The nucleus is the battleground between the strong nuclear (only active at a distance of a small nucleus) and the electromagnetic forces. As you move up the period table toward iron, you find that the nuclei have more nucleons, al ...
... Why is it that the middle elements have the highest binding energies? The nucleus is the battleground between the strong nuclear (only active at a distance of a small nucleus) and the electromagnetic forces. As you move up the period table toward iron, you find that the nuclei have more nucleons, al ...
Radioactive Decay
... represent completed nuclear energy levels ~ 2, 8, 20, 28, 50, 82 and 126 are called Magic Numbers. ...
... represent completed nuclear energy levels ~ 2, 8, 20, 28, 50, 82 and 126 are called Magic Numbers. ...
Radioactivity Revision Questions Decay – Nucleus
... Sometimes the nucleus of an atom is unstable. A change will occur in the nucleus to make it more stable. The change is called a decay 2. During Radioactive Decay, what can a Nucleus Emit? When a nucleus decays it will emit (give out) some particles or waves. Emitting particles or waves from the nucl ...
... Sometimes the nucleus of an atom is unstable. A change will occur in the nucleus to make it more stable. The change is called a decay 2. During Radioactive Decay, what can a Nucleus Emit? When a nucleus decays it will emit (give out) some particles or waves. Emitting particles or waves from the nucl ...
Chapter 29
... • These forces should cause the nucleus to fly apart • The nuclei are stable because of the presence of another, short-range force, called the nuclear force • This is an attractive force that acts between all nuclear particles • The nuclear attractive force is stronger than the Coulomb repulsive for ...
... • These forces should cause the nucleus to fly apart • The nuclei are stable because of the presence of another, short-range force, called the nuclear force • This is an attractive force that acts between all nuclear particles • The nuclear attractive force is stronger than the Coulomb repulsive for ...
Beyond Element 83 are very unstable (radioactive)
... unstable (radioactive) • No amount neutrons can hold nucleus together once it has 83+ protons • All Elements 83 and above on PT are radioactive • Other elements may have radioactive isotopes applet ...
... unstable (radioactive) • No amount neutrons can hold nucleus together once it has 83+ protons • All Elements 83 and above on PT are radioactive • Other elements may have radioactive isotopes applet ...
Radioactivity - Williamstown Independent Schools
... • Radiation is emitted by almost everything. Rocks, soil, the air and living things all contain radioactive elements. We are exposed to radiation from the sun, distant stars and from our own planet. • In addition, nuclear testing has added radiation to our environment. ...
... • Radiation is emitted by almost everything. Rocks, soil, the air and living things all contain radioactive elements. We are exposed to radiation from the sun, distant stars and from our own planet. • In addition, nuclear testing has added radiation to our environment. ...
3 main types of particle
... Name the 2 key items involved What is the relationship between the rate of ...
... Name the 2 key items involved What is the relationship between the rate of ...
nuclear chemistry - Magoffin County Schools
... pursue the creation of an atomic weapon. The result was the creation of two very powerful atomic bombs named FAT MAN and LITTLE BOY which were used against the cities of HIROSHIMA and NAGASAKI, JAPAN, effectively brining the World War II to an end. In this unit, we will explore the basic principles ...
... pursue the creation of an atomic weapon. The result was the creation of two very powerful atomic bombs named FAT MAN and LITTLE BOY which were used against the cities of HIROSHIMA and NAGASAKI, JAPAN, effectively brining the World War II to an end. In this unit, we will explore the basic principles ...
Radioactive decay
Radioactive decay, also known as nuclear decay or radioactivity, is the process by which a nucleus of an unstable atom loses energy by emitting radiation. A material that spontaneously emits such radiation — which includes alpha particles, beta particles, gamma rays and conversion electrons — is considered radioactive.Radioactive decay is a stochastic (i.e. random) process at the level of single atoms, in that, according to quantum theory, it is impossible to predict when a particular atom will decay. The chance that a given atom will decay never changes, that is, it does not matter how long the atom has existed. For a large collection of atoms however, the decay rate for that collection can be calculated from their measured decay constants or half-lives. This is the basis of radiometric dating. The half-lives of radioactive atoms have no known limits for shortness or length of duration, and range over 55 orders of magnitude in time.There are many types of radioactive decay (see table below). A decay, or loss of energy from the nucleus, results when an atom with one type of nucleus, called the parent radionuclide (or parent radioisotope), transforms into an atom with a nucleus in a different state, or with a nucleus containing a different number of protons and neutrons. The product is called the daughter nuclide. In some decays, the parent and the daughter nuclides are different chemical elements, and thus the decay process results in the creation of an atom of a different element. This is known as a nuclear transmutation.The first decay processes to be discovered were alpha decay, beta decay, and gamma decay. Alpha decay occurs when the nucleus ejects an alpha particle (helium nucleus). This is the most common process of emitting nucleons, but in rarer types of decays, nuclei can eject protons, or in the case of cluster decay specific nuclei of other elements. Beta decay occurs when the nucleus emits an electron or positron and a neutrino, in a process that changes a proton to a neutron or the other way about. The nucleus may capture an orbiting electron, causing a proton to convert into a neutron in a process called electron capture. All of these processes result in a well-defined nuclear transmutation.By contrast, there are radioactive decay processes that do not result in a nuclear transmutation. The energy of an excited nucleus may be emitted as a gamma ray in a process called gamma decay, or be used to eject an orbital electron by its interaction with the excited nucleus, in a process called internal conversion. Highly excited neutron-rich nuclei, formed as the product of other types of decay, occasionally lose energy by way of neutron emission, resulting in a change of an element from one isotope to another. Another type of radioactive decay results in products that are not defined, but appear in a range of ""pieces"" of the original nucleus. This decay, called spontaneous fission, happens when a large unstable nucleus spontaneously splits into two (and occasionally three) smaller daughter nuclei, and generally leads to the emission of gamma rays, neutrons, or other particles from those products.For a summary table showing the number of stable and radioactive nuclides in each category, see radionuclide. There exist twenty-nine chemical elements on Earth that are radioactive. They are those that contain thirty-four radionuclides that date before the time of formation of the solar system, and are known as primordial nuclides. Well-known examples are uranium and thorium, but also included are naturally occurring long-lived radioisotopes such as potassium-40. Another fifty or so shorter-lived radionuclides, such as radium and radon, found on Earth, are the products of decay chains that began with the primordial nuclides, and ongoing cosmogenic processes, such as the production of carbon-14 from nitrogen-14 by cosmic rays. Radionuclides may also be produced artificially in particle accelerators or nuclear reactors, resulting in 650 of these with half-lives of over an hour, and several thousand more with even shorter half-lives. See this list of nuclides for a list of these, sorted by half life.