31.1 Nuclear Structure
... Example 3 The Binding Energy of the Helium Nucleus Revisited The atomic mass of helium is 4.0026u and the atomic mass of 1hydrogen isotope is 1.0078u. Using atomic mass units, instead of kilograms, obtain the binding energy of the helium nucleus. ...
... Example 3 The Binding Energy of the Helium Nucleus Revisited The atomic mass of helium is 4.0026u and the atomic mass of 1hydrogen isotope is 1.0078u. Using atomic mass units, instead of kilograms, obtain the binding energy of the helium nucleus. ...
By what process do most stars release energy? A. Electromagnetic
... Nuclear energy can be generated by ssion or fusion. Fusion is not currently being used in reactors as an energy source. Why is this? A. ...
... Nuclear energy can be generated by ssion or fusion. Fusion is not currently being used in reactors as an energy source. Why is this? A. ...
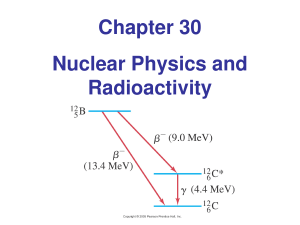
Chapter 30 Nuclear Physics and Radioactivity
... The total mass of a stable nucleus is always less than the sum of the masses of its separate pieces; the protons and neutrons. Where has the mass gone? Energy, as radiation or kinetic energy, is released during formation of a nucleus by ‘fusion’ of smaller nuclei, giving a net mass difference. T ...
... The total mass of a stable nucleus is always less than the sum of the masses of its separate pieces; the protons and neutrons. Where has the mass gone? Energy, as radiation or kinetic energy, is released during formation of a nucleus by ‘fusion’ of smaller nuclei, giving a net mass difference. T ...
Nuclear Chemistry
... interested in Becquereal's discovery. While experimenting with their own uranium-containing ore, they came up with the term "radioactivity" to describe the spontaneous emissions that they studied. This word is still used today to describe this special characteristic of some elements.(radioisotopes). ...
... interested in Becquereal's discovery. While experimenting with their own uranium-containing ore, they came up with the term "radioactivity" to describe the spontaneous emissions that they studied. This word is still used today to describe this special characteristic of some elements.(radioisotopes). ...
Chapter 32 Nuclear Physics
... The nucleus of an atom is incredibly small. The diameter of the nucleus is about 100,000 times smaller than the diameter of the atom. Although small, the nucleus contains more than 99.9% of the mass of an atom, because each of its constitutes (protons and neutrons) is about 1800 times more massive t ...
... The nucleus of an atom is incredibly small. The diameter of the nucleus is about 100,000 times smaller than the diameter of the atom. Although small, the nucleus contains more than 99.9% of the mass of an atom, because each of its constitutes (protons and neutrons) is about 1800 times more massive t ...
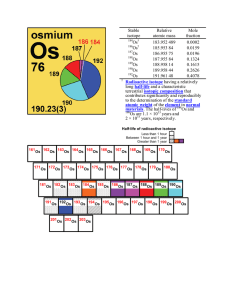
Stable isotope Relative atomic mass Mole fraction Os 183.952 489
... either positive or negative charge (an electron or positron). [return] electron – elementary particle of matter with a negative electric charge and a rest mass of about 9.109 × 10–31 kg. element (chemical element) – a species of atoms; all atoms with the same number of protons in the atomic nucleus. ...
... either positive or negative charge (an electron or positron). [return] electron – elementary particle of matter with a negative electric charge and a rest mass of about 9.109 × 10–31 kg. element (chemical element) – a species of atoms; all atoms with the same number of protons in the atomic nucleus. ...
Chapter 21 - Richsingiser.com
... 5 The zone of stability contains all of the stable nuclides, but some nuclides in this band are unstable. 6 Tc (Z=43), Pm (Z=61), and all elements beyond Bi (Z=83) have no stable isotopes. ...
... 5 The zone of stability contains all of the stable nuclides, but some nuclides in this band are unstable. 6 Tc (Z=43), Pm (Z=61), and all elements beyond Bi (Z=83) have no stable isotopes. ...
Modern Physics - hrsbstaff.ednet.ns.ca
... Isotopes of a given element correspond to nuclei with different numbers of neutrons. This results in a variety of different properties for the nuclei, including the obvious one of mass. The chemical behavior, however, is governed by the lectrons. All isotopes of a given element have the same number ...
... Isotopes of a given element correspond to nuclei with different numbers of neutrons. This results in a variety of different properties for the nuclei, including the obvious one of mass. The chemical behavior, however, is governed by the lectrons. All isotopes of a given element have the same number ...
IB-ATOMIC-AND-NUCLEAR-PHYSICS-DEFINITIONS
... NUCLEON: The particles in the nucleus (proton or neutron). NUCLEON NUMBER, A: The number of nucleons in the nucleus. PROTON NUMBER, Z: The number of protons in the nucleus. NEUTRON NUMBER, N: N=A-Z RADIOACTIVE DECAY: A random and spontaneous process in which unstable nuclei emit a particle (disinteg ...
... NUCLEON: The particles in the nucleus (proton or neutron). NUCLEON NUMBER, A: The number of nucleons in the nucleus. PROTON NUMBER, Z: The number of protons in the nucleus. NEUTRON NUMBER, N: N=A-Z RADIOACTIVE DECAY: A random and spontaneous process in which unstable nuclei emit a particle (disinteg ...
SMP Quiz Session 1
... produce lighter nuclei and energy. 2) The combining of electrons with nuclei to produce atoms and release energy. 3) The process of fusing together light nuclei (for example, hydrogen) to produce heav ...
... produce lighter nuclei and energy. 2) The combining of electrons with nuclei to produce atoms and release energy. 3) The process of fusing together light nuclei (for example, hydrogen) to produce heav ...
Nuclear Radiation1516
... When a nucleus fissions, it splits into several smaller fragments. These fragments, or fission products, are about equal to half the original mass. Two or three neutrons are also emitted. The sum of the masses of these fragments is less than the original mass. This 'missing' mass (about 0.1 percent ...
... When a nucleus fissions, it splits into several smaller fragments. These fragments, or fission products, are about equal to half the original mass. Two or three neutrons are also emitted. The sum of the masses of these fragments is less than the original mass. This 'missing' mass (about 0.1 percent ...
Chapter 29: Nuclear Physics
... Gamma rays were determined to be high energy photons. A gamma ray will be emitted when a nucleus is an excited state when making a transition to a lower energy level. For example, ...
... Gamma rays were determined to be high energy photons. A gamma ray will be emitted when a nucleus is an excited state when making a transition to a lower energy level. For example, ...
Half-life and Radioactive Decay guided notes
... substance remaining decreases over time. The amount of time it takes for only half of the original sample to remain is equal to the amount it takes for the radioactive sample to decrease from one-half to one-quarter of the original amount. For example, if it took twenty days for the sample to decrea ...
... substance remaining decreases over time. The amount of time it takes for only half of the original sample to remain is equal to the amount it takes for the radioactive sample to decrease from one-half to one-quarter of the original amount. For example, if it took twenty days for the sample to decrea ...
Radioactive Decay Series
... nuclide of each decay series. The daughter nuclides are the nuclides or new elements that are produced by decay of the parent nuclide. All naturally occurring nuclides with atomic numbers greater than 83 are radioactive ...
... nuclide of each decay series. The daughter nuclides are the nuclides or new elements that are produced by decay of the parent nuclide. All naturally occurring nuclides with atomic numbers greater than 83 are radioactive ...
Student 5
... beta or gamma radiation. The two principles observed is the conservation of Mass number, and the conservation atomic number. The number of protons and neutrons remain the same for conservation of Mass number. The charge remains the same, for conservation atomic number. [2] What are the products of n ...
... beta or gamma radiation. The two principles observed is the conservation of Mass number, and the conservation atomic number. The number of protons and neutrons remain the same for conservation of Mass number. The charge remains the same, for conservation atomic number. [2] What are the products of n ...
1 The Nucleus Total number of nucleons: mass number Number of
... “Well, we make drugs. Like statins. Very useful. They are inhibitors of a protein called HMGA-CoA reductase, and they help to control cholesterol biosynthesis and limit cardiovascular ...
... “Well, we make drugs. Like statins. Very useful. They are inhibitors of a protein called HMGA-CoA reductase, and they help to control cholesterol biosynthesis and limit cardiovascular ...
Radioactivity and Nuclear Reactions
... • When an atom loses an alpha particle, it no longer has the same number of protons, so it no longer is the same element. • Transmutation is the process of changing one element to another through nuclear decay. ...
... • When an atom loses an alpha particle, it no longer has the same number of protons, so it no longer is the same element. • Transmutation is the process of changing one element to another through nuclear decay. ...
Nuclear Chemistry
... some are beta decay - the stable end point is an element with atomic # less than 83 (lead) - there are also unstable lead isotopes which are intermediates ...
... some are beta decay - the stable end point is an element with atomic # less than 83 (lead) - there are also unstable lead isotopes which are intermediates ...
Nuclear Notation
... photographic paper. – When developed the film had an image on it from the Uranium decaying ...
... photographic paper. – When developed the film had an image on it from the Uranium decaying ...
SCIENCE 10: (7.1) ATOMIC THEORY, ISOTOPES
... Radioactive atoms release energy until they become __________, often as different atoms. Ex. Uranium -238 undergoes 14 different radioactive decay steps before forming stable lead-206. ...
... Radioactive atoms release energy until they become __________, often as different atoms. Ex. Uranium -238 undergoes 14 different radioactive decay steps before forming stable lead-206. ...
Radioactivity
... • You can heat the substance up, or subject it to high pressure or strong magnetic fields - in fact, do whatever you like to it - and you won't affect the rate of decay in the slightest. ...
... • You can heat the substance up, or subject it to high pressure or strong magnetic fields - in fact, do whatever you like to it - and you won't affect the rate of decay in the slightest. ...
Document
... number of nucleons as the parent, but the atomic number is one less In addition, an electron (positron) was observed The emission of the electron is from the nucleus ...
... number of nucleons as the parent, but the atomic number is one less In addition, an electron (positron) was observed The emission of the electron is from the nucleus ...
Chp 7.1 Atomic Theory and Radioactive Decay
... • Natural background radiation exists all around us. • This radiation consists of high energy particles or waves being emitted from a variety of materials. ...
... • Natural background radiation exists all around us. • This radiation consists of high energy particles or waves being emitted from a variety of materials. ...
Radiation_What Is It
... During negative beta decay, excess neutrons are converted into protons, electrons, and antineutrinos. The protons remain in the nucleus but the new electrons are emitted as negative beta particles (ß-) or negatrons. You may wish to think of them as “nuclear electrons.” ...
... During negative beta decay, excess neutrons are converted into protons, electrons, and antineutrinos. The protons remain in the nucleus but the new electrons are emitted as negative beta particles (ß-) or negatrons. You may wish to think of them as “nuclear electrons.” ...
Radioactive decay
Radioactive decay, also known as nuclear decay or radioactivity, is the process by which a nucleus of an unstable atom loses energy by emitting radiation. A material that spontaneously emits such radiation — which includes alpha particles, beta particles, gamma rays and conversion electrons — is considered radioactive.Radioactive decay is a stochastic (i.e. random) process at the level of single atoms, in that, according to quantum theory, it is impossible to predict when a particular atom will decay. The chance that a given atom will decay never changes, that is, it does not matter how long the atom has existed. For a large collection of atoms however, the decay rate for that collection can be calculated from their measured decay constants or half-lives. This is the basis of radiometric dating. The half-lives of radioactive atoms have no known limits for shortness or length of duration, and range over 55 orders of magnitude in time.There are many types of radioactive decay (see table below). A decay, or loss of energy from the nucleus, results when an atom with one type of nucleus, called the parent radionuclide (or parent radioisotope), transforms into an atom with a nucleus in a different state, or with a nucleus containing a different number of protons and neutrons. The product is called the daughter nuclide. In some decays, the parent and the daughter nuclides are different chemical elements, and thus the decay process results in the creation of an atom of a different element. This is known as a nuclear transmutation.The first decay processes to be discovered were alpha decay, beta decay, and gamma decay. Alpha decay occurs when the nucleus ejects an alpha particle (helium nucleus). This is the most common process of emitting nucleons, but in rarer types of decays, nuclei can eject protons, or in the case of cluster decay specific nuclei of other elements. Beta decay occurs when the nucleus emits an electron or positron and a neutrino, in a process that changes a proton to a neutron or the other way about. The nucleus may capture an orbiting electron, causing a proton to convert into a neutron in a process called electron capture. All of these processes result in a well-defined nuclear transmutation.By contrast, there are radioactive decay processes that do not result in a nuclear transmutation. The energy of an excited nucleus may be emitted as a gamma ray in a process called gamma decay, or be used to eject an orbital electron by its interaction with the excited nucleus, in a process called internal conversion. Highly excited neutron-rich nuclei, formed as the product of other types of decay, occasionally lose energy by way of neutron emission, resulting in a change of an element from one isotope to another. Another type of radioactive decay results in products that are not defined, but appear in a range of ""pieces"" of the original nucleus. This decay, called spontaneous fission, happens when a large unstable nucleus spontaneously splits into two (and occasionally three) smaller daughter nuclei, and generally leads to the emission of gamma rays, neutrons, or other particles from those products.For a summary table showing the number of stable and radioactive nuclides in each category, see radionuclide. There exist twenty-nine chemical elements on Earth that are radioactive. They are those that contain thirty-four radionuclides that date before the time of formation of the solar system, and are known as primordial nuclides. Well-known examples are uranium and thorium, but also included are naturally occurring long-lived radioisotopes such as potassium-40. Another fifty or so shorter-lived radionuclides, such as radium and radon, found on Earth, are the products of decay chains that began with the primordial nuclides, and ongoing cosmogenic processes, such as the production of carbon-14 from nitrogen-14 by cosmic rays. Radionuclides may also be produced artificially in particle accelerators or nuclear reactors, resulting in 650 of these with half-lives of over an hour, and several thousand more with even shorter half-lives. See this list of nuclides for a list of these, sorted by half life.