Nuclear Chemistry powerpoint
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
File
... For smaller nuclei (Z 20) stable nuclei have a neutron-to-proton ratio close to 1:1. As nuclei get larger, it takes a greater number of neutrons to stabilize the nucleus. The shaded region in the ...
... For smaller nuclei (Z 20) stable nuclei have a neutron-to-proton ratio close to 1:1. As nuclei get larger, it takes a greater number of neutrons to stabilize the nucleus. The shaded region in the ...
Nuclear Chemistry powerpoint
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
Average Atomic Mass
... one element changing into an atom of another element. These reactions, which involve a change in an atom’s nucleus, are called nuclear reactions. ...
... one element changing into an atom of another element. These reactions, which involve a change in an atom’s nucleus, are called nuclear reactions. ...
Chapter 30: Nuclear Physics What will we learn in this chapter?
... Can be shielded with thick clothing, used in medical applications. ...
... Can be shielded with thick clothing, used in medical applications. ...
Chapter 37
... • Important factors for stable isotopesnuclear stability correlates with: – Ratio of neutrons to protons in the isotope. – Nuclei with large number of protons (84 or more) tend to be unstable. – The “magic numbers” of 2, 8, 20, 50, 82, or 126 help determine stability. These numbers of protons or ne ...
... • Important factors for stable isotopesnuclear stability correlates with: – Ratio of neutrons to protons in the isotope. – Nuclei with large number of protons (84 or more) tend to be unstable. – The “magic numbers” of 2, 8, 20, 50, 82, or 126 help determine stability. These numbers of protons or ne ...
Masses in Atomic Units - proton 1.007 u 938.28 MeV
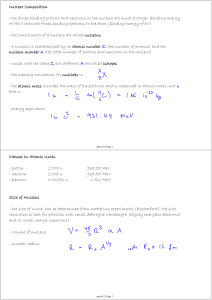
... - the forces binding protons and neutrons in the nucleus are much stronger (binding energy of MeV) than the forces binding electrons to the atom (binding energy of eV) - the constituents of a nucleus are called nucleons - a nucleus is characterized by its atomic number Z (the number of protons) and ...
... - the forces binding protons and neutrons in the nucleus are much stronger (binding energy of MeV) than the forces binding electrons to the atom (binding energy of eV) - the constituents of a nucleus are called nucleons - a nucleus is characterized by its atomic number Z (the number of protons) and ...
Nuclear Chemistry powerpoint
... γ has no mass ( ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
... γ has no mass ( ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
Isotopes and Radioactive Decay
... GAMMA RADIATION: Radiation that is made up of ________________________ rays. A gamma ray is high-energy and contains no _____________ and is represented by the symbol __________. Gamma rays usually accompany ___________________ and ___________________ radiation. Gamma rays also account for _________ ...
... GAMMA RADIATION: Radiation that is made up of ________________________ rays. A gamma ray is high-energy and contains no _____________ and is represented by the symbol __________. Gamma rays usually accompany ___________________ and ___________________ radiation. Gamma rays also account for _________ ...
Chapter 28 for Chem
... Closely related is “positron emission,” also called Beta-plus decay. This occurs when a proton in the nucleus splits into a neutron and a positron. A positron has a positive charge and is about the same mass as an electron. (Z goes down by 1; mass stays the same.) ...
... Closely related is “positron emission,” also called Beta-plus decay. This occurs when a proton in the nucleus splits into a neutron and a positron. A positron has a positive charge and is about the same mass as an electron. (Z goes down by 1; mass stays the same.) ...
AP Chem
... 25. Read about Japanese nuclear power plant accident. Write facts about the accident and current effects. ( ½ page) ...
... 25. Read about Japanese nuclear power plant accident. Write facts about the accident and current effects. ( ½ page) ...
nuclear chemistry - Wood County Schools
... and continually split and fuse it to produce energy. Iron (Fe, number 26) has the highest energy stored in its nucleus. Atoms larger than iron will release energy when they undergo fission, and atoms smaller than iron will release energy when they undergo fusion. ...
... and continually split and fuse it to produce energy. Iron (Fe, number 26) has the highest energy stored in its nucleus. Atoms larger than iron will release energy when they undergo fission, and atoms smaller than iron will release energy when they undergo fusion. ...
Radioactivity2015
... Unstable atoms are said to be radioactive. In order to reach stability, these atoms give off, or emit, the excess energy or mass. These emissions are called radiation. ...
... Unstable atoms are said to be radioactive. In order to reach stability, these atoms give off, or emit, the excess energy or mass. These emissions are called radiation. ...
Physics: Principles and Applications, 6e Giancoli
... 5) Compared to the masses of its separate protons and neutrons, the total mass of a stable nucleus is always A) less. B) the same. C) greater. D) zero. 6) When nucleons join to form a stable nucleus, energy is A) destroyed. B) absorbed. C) released. D) not transferred. 7) The binding energy of a nuc ...
... 5) Compared to the masses of its separate protons and neutrons, the total mass of a stable nucleus is always A) less. B) the same. C) greater. D) zero. 6) When nucleons join to form a stable nucleus, energy is A) destroyed. B) absorbed. C) released. D) not transferred. 7) The binding energy of a nuc ...
Chapter #20 Nuclear Chemistry
... together by overcoming the repulsive force of the protons. Neutrons are present to help dissipate the repulsive forces between the protons As the atomic number (number of protons) increases, so does the number of neutrons to shield the repulsion of the protons All nuclides with 84 or more protons ar ...
... together by overcoming the repulsive force of the protons. Neutrons are present to help dissipate the repulsive forces between the protons As the atomic number (number of protons) increases, so does the number of neutrons to shield the repulsion of the protons All nuclides with 84 or more protons ar ...
Independent Study: Nuclear Chemistry
... 9. When an uranium atom is subjected to neutron bombardment, the atom is a. destroyed b. disintegrated c. ionized d. split 10. When a uranium-238 atom decays, it emits an alpha particle. The atomic mass of the new atom formed is a. decreased by 2 b. decreased by 4 c. increased by 4 d. increased by ...
... 9. When an uranium atom is subjected to neutron bombardment, the atom is a. destroyed b. disintegrated c. ionized d. split 10. When a uranium-238 atom decays, it emits an alpha particle. The atomic mass of the new atom formed is a. decreased by 2 b. decreased by 4 c. increased by 4 d. increased by ...
Independent Study: Nuclear Chemistry
... 9. When an uranium atom is subjected to neutron bombardment, the atom is a. destroyed b. disintegrated c. ionized d. split 10. When a uranium-238 atom decays, it emits an alpha particle. The atomic mass of the new atom formed is a. decreased by 2 b. decreased by 4 c. increased by 4 d. increased by ...
... 9. When an uranium atom is subjected to neutron bombardment, the atom is a. destroyed b. disintegrated c. ionized d. split 10. When a uranium-238 atom decays, it emits an alpha particle. The atomic mass of the new atom formed is a. decreased by 2 b. decreased by 4 c. increased by 4 d. increased by ...
Atomic/Nuclear
... When a gamma ray is released, the same number of neutrons and protons are present but they have settled to a lower energy level. Alpha particles are stopped by air molecules in a few centimeters. Beta particles are stopped by air molecules in a few meters. Gamma rays can travel very long distances t ...
... When a gamma ray is released, the same number of neutrons and protons are present but they have settled to a lower energy level. Alpha particles are stopped by air molecules in a few centimeters. Beta particles are stopped by air molecules in a few meters. Gamma rays can travel very long distances t ...
Revision of Atomic Structure and Nuclide Notations Nuclide
... The atoms of most elements have isotopes. Some of these isotopes have nuclei which are unstable and give out different types of particles or rays. This release (emission) of particles and rays is what we call radiation and helps to make the atom more stable. Radioactivity is all around us. We are co ...
... The atoms of most elements have isotopes. Some of these isotopes have nuclei which are unstable and give out different types of particles or rays. This release (emission) of particles and rays is what we call radiation and helps to make the atom more stable. Radioactivity is all around us. We are co ...
Nuclear Fission vs Fusion
... Nuclear Fusion: Source of energy in the Sun that produces heat from the fusing of elements like hydrogen. Produces unsurpassed quantities of energy. Does not produce particulate air pollution like fossil fuels and coal. Does not produce a radioactive waste product that will need to be stored. Curren ...
... Nuclear Fusion: Source of energy in the Sun that produces heat from the fusing of elements like hydrogen. Produces unsurpassed quantities of energy. Does not produce particulate air pollution like fossil fuels and coal. Does not produce a radioactive waste product that will need to be stored. Curren ...
Nuclear Fission vs. Nuclear Fusion
... Nuclear Fusion: Source of energy in the Sun that produces heat from the fusing of elements like hydrogen. Produces unsurpassed quantities of energy. Does not produce particulate air pollution like fossil fuels and coal. Does not produce a radioactive waste product that will need to be stored. Curren ...
... Nuclear Fusion: Source of energy in the Sun that produces heat from the fusing of elements like hydrogen. Produces unsurpassed quantities of energy. Does not produce particulate air pollution like fossil fuels and coal. Does not produce a radioactive waste product that will need to be stored. Curren ...
(or radioactive isotopes).
... Atoms of the same element containing the same number of protons but different numbers of neutrons in their nuclei. Because they have the same number of electrons there is NO difference to their chemical behaviour. ...
... Atoms of the same element containing the same number of protons but different numbers of neutrons in their nuclei. Because they have the same number of electrons there is NO difference to their chemical behaviour. ...
Nuclear Chemistry - Mrs. Carlyle`s Classroom
... Atomic nuclei are made of protons and neutrons, which are collectively called nucleons Nuclear radiation – particles or electromagnetic radiation emitted from the nucleus during radioactive decay Radioactive nuclide – an unstable nucleus that undergoes radioactive decay Nuclear reaction – a r ...
... Atomic nuclei are made of protons and neutrons, which are collectively called nucleons Nuclear radiation – particles or electromagnetic radiation emitted from the nucleus during radioactive decay Radioactive nuclide – an unstable nucleus that undergoes radioactive decay Nuclear reaction – a r ...
Radioactive decay
Radioactive decay, also known as nuclear decay or radioactivity, is the process by which a nucleus of an unstable atom loses energy by emitting radiation. A material that spontaneously emits such radiation — which includes alpha particles, beta particles, gamma rays and conversion electrons — is considered radioactive.Radioactive decay is a stochastic (i.e. random) process at the level of single atoms, in that, according to quantum theory, it is impossible to predict when a particular atom will decay. The chance that a given atom will decay never changes, that is, it does not matter how long the atom has existed. For a large collection of atoms however, the decay rate for that collection can be calculated from their measured decay constants or half-lives. This is the basis of radiometric dating. The half-lives of radioactive atoms have no known limits for shortness or length of duration, and range over 55 orders of magnitude in time.There are many types of radioactive decay (see table below). A decay, or loss of energy from the nucleus, results when an atom with one type of nucleus, called the parent radionuclide (or parent radioisotope), transforms into an atom with a nucleus in a different state, or with a nucleus containing a different number of protons and neutrons. The product is called the daughter nuclide. In some decays, the parent and the daughter nuclides are different chemical elements, and thus the decay process results in the creation of an atom of a different element. This is known as a nuclear transmutation.The first decay processes to be discovered were alpha decay, beta decay, and gamma decay. Alpha decay occurs when the nucleus ejects an alpha particle (helium nucleus). This is the most common process of emitting nucleons, but in rarer types of decays, nuclei can eject protons, or in the case of cluster decay specific nuclei of other elements. Beta decay occurs when the nucleus emits an electron or positron and a neutrino, in a process that changes a proton to a neutron or the other way about. The nucleus may capture an orbiting electron, causing a proton to convert into a neutron in a process called electron capture. All of these processes result in a well-defined nuclear transmutation.By contrast, there are radioactive decay processes that do not result in a nuclear transmutation. The energy of an excited nucleus may be emitted as a gamma ray in a process called gamma decay, or be used to eject an orbital electron by its interaction with the excited nucleus, in a process called internal conversion. Highly excited neutron-rich nuclei, formed as the product of other types of decay, occasionally lose energy by way of neutron emission, resulting in a change of an element from one isotope to another. Another type of radioactive decay results in products that are not defined, but appear in a range of ""pieces"" of the original nucleus. This decay, called spontaneous fission, happens when a large unstable nucleus spontaneously splits into two (and occasionally three) smaller daughter nuclei, and generally leads to the emission of gamma rays, neutrons, or other particles from those products.For a summary table showing the number of stable and radioactive nuclides in each category, see radionuclide. There exist twenty-nine chemical elements on Earth that are radioactive. They are those that contain thirty-four radionuclides that date before the time of formation of the solar system, and are known as primordial nuclides. Well-known examples are uranium and thorium, but also included are naturally occurring long-lived radioisotopes such as potassium-40. Another fifty or so shorter-lived radionuclides, such as radium and radon, found on Earth, are the products of decay chains that began with the primordial nuclides, and ongoing cosmogenic processes, such as the production of carbon-14 from nitrogen-14 by cosmic rays. Radionuclides may also be produced artificially in particle accelerators or nuclear reactors, resulting in 650 of these with half-lives of over an hour, and several thousand more with even shorter half-lives. See this list of nuclides for a list of these, sorted by half life.