Atoms and the Periodic Table
... c. Unlike protons and electrons, neutrons have no charge. d. Protons and neutrons have the same charge. 32. This Group 2 element has fewer protons than bromine, but no more protons than sulfur. a. Potassium ...
... c. Unlike protons and electrons, neutrons have no charge. d. Protons and neutrons have the same charge. 32. This Group 2 element has fewer protons than bromine, but no more protons than sulfur. a. Potassium ...
Darlington High School EDI Lesson Plan Teacher: L. Grooms
... PS2.1 Compare the subatomic particles, protons, neutrons and electrons in regard to the mass, location, and charge and explain how these particles affect the properties of an atom. PS 2.3 Explain the trends of the periodic table based on the elements’ valence electrons and atomic number. PS 2.4 Use ...
... PS2.1 Compare the subatomic particles, protons, neutrons and electrons in regard to the mass, location, and charge and explain how these particles affect the properties of an atom. PS 2.3 Explain the trends of the periodic table based on the elements’ valence electrons and atomic number. PS 2.4 Use ...
1 - Hobbs Freshman High School
... 11. In an experiment Rutherford bombarded gold foil with high-speed positively charges particles and found, that while most of the particles passed through this foil, a few bounced back. In terms of atomic structure, which of the following statements explains this observation? (The atom is mostly em ...
... 11. In an experiment Rutherford bombarded gold foil with high-speed positively charges particles and found, that while most of the particles passed through this foil, a few bounced back. In terms of atomic structure, which of the following statements explains this observation? (The atom is mostly em ...
Unit Nuclear Chemistry
... › As the atomic number increases, stable nuclei have neutronproton ratios greater than a 1 to 1 and become less stable › Ex 206 82 Pb has a ratio of 124/82 (1.51N to 1 P) › The stability of a nucleus also depends on the even-odd relationship of protons and neutrons ...
... › As the atomic number increases, stable nuclei have neutronproton ratios greater than a 1 to 1 and become less stable › Ex 206 82 Pb has a ratio of 124/82 (1.51N to 1 P) › The stability of a nucleus also depends on the even-odd relationship of protons and neutrons ...
Physics 100 Group Session for Chapters 1 – 3
... (c) On the basis of your answers, which nucleus requires more energy to disassemble? (mp = 1.007 825 u and mn = 1.008 665 u ) REASONING The mass defect is the total mass of the stationary separated nucleons (protons and neutrons) minus the mass of the intact nucleus. The given atomic masses are for ...
... (c) On the basis of your answers, which nucleus requires more energy to disassemble? (mp = 1.007 825 u and mn = 1.008 665 u ) REASONING The mass defect is the total mass of the stationary separated nucleons (protons and neutrons) minus the mass of the intact nucleus. The given atomic masses are for ...
Atoms and Elements
... John Dalton (1766-1844) proposed an ___________________________ While this theory was __________completely correct, it revolutionized how chemists looked at matter and brought about chemistry as we know it ___________________instead of alchemy Thus, it’s an important __________________in the history ...
... John Dalton (1766-1844) proposed an ___________________________ While this theory was __________completely correct, it revolutionized how chemists looked at matter and brought about chemistry as we know it ___________________instead of alchemy Thus, it’s an important __________________in the history ...
Atoms
... • Equal to the atomic number of the atom • Contribute to the atomic mass • Equal to the number of electrons ...
... • Equal to the atomic number of the atom • Contribute to the atomic mass • Equal to the number of electrons ...
atom`s - Hauppauge School District
... moved from one energy level to another when they gained energy • They released the energy as light (photons) • In the lowest levels, or the ground state, to the excited state ____________________ ________________________ • When electrons moved from the excited state back to the ground state, _______ ...
... moved from one energy level to another when they gained energy • They released the energy as light (photons) • In the lowest levels, or the ground state, to the excited state ____________________ ________________________ • When electrons moved from the excited state back to the ground state, _______ ...
Chapter 4
... d. Cathode rays were found to be made of protons. All atoms are ____. a. positively charged, with the number of protons exceeding the number of electrons b. negatively charged, with the number of electrons exceeding the number of protons c. neutral, with the number of protons equaling the number of ...
... d. Cathode rays were found to be made of protons. All atoms are ____. a. positively charged, with the number of protons exceeding the number of electrons b. negatively charged, with the number of electrons exceeding the number of protons c. neutral, with the number of protons equaling the number of ...
Chapter 4 – Structure of the Atom
... In the early 1800’s, John Dalton (1766-1844) studied many chemical reactions and proposed Dalton’s Atomic Theory. a) All matter is composed of very small particles called ATOMS. b) Atoms of the same elements are identical. c) Atoms cannot be created, divided or destroyed. d) Different atoms combine ...
... In the early 1800’s, John Dalton (1766-1844) studied many chemical reactions and proposed Dalton’s Atomic Theory. a) All matter is composed of very small particles called ATOMS. b) Atoms of the same elements are identical. c) Atoms cannot be created, divided or destroyed. d) Different atoms combine ...
Chapter 16 Notes - Mr. Julien`s Homepage
... 2. Paper, clothing, and skin will protect you from alpha particles. 3. Beta particles have a very small mass and move much faster and farther than alpha particles, traveling as much as several meters through air. 4. Beta particles can penetrate as far as 4-5 mm into the body, burning the surface of ...
... 2. Paper, clothing, and skin will protect you from alpha particles. 3. Beta particles have a very small mass and move much faster and farther than alpha particles, traveling as much as several meters through air. 4. Beta particles can penetrate as far as 4-5 mm into the body, burning the surface of ...
Name
... element and the relative abundance of its isotopes. a. In nature, most elements occur as a mixture of two or more isotopes. b. Isotopes of an element do not have a specific natural percent abundance. c. The average atomic mass of an element is usually closest to that of the isotope with the highest ...
... element and the relative abundance of its isotopes. a. In nature, most elements occur as a mixture of two or more isotopes. b. Isotopes of an element do not have a specific natural percent abundance. c. The average atomic mass of an element is usually closest to that of the isotope with the highest ...
5.3.1 The Nuclear Atom
... was surprised that some alpha particles were deflected slightly or bounced back. The ‘plum pudding’ model could not explain these results, so Rutherford proposed his ‘nuclear’ model of the atom. He suggested that an atom is mostly empty space with its positive charge and most of its mass in a tiny c ...
... was surprised that some alpha particles were deflected slightly or bounced back. The ‘plum pudding’ model could not explain these results, so Rutherford proposed his ‘nuclear’ model of the atom. He suggested that an atom is mostly empty space with its positive charge and most of its mass in a tiny c ...
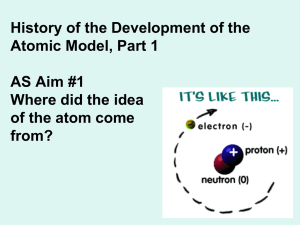
History and atomic structure
... His ideas carried through middle ages. Alchemists change lead to gold ...
... His ideas carried through middle ages. Alchemists change lead to gold ...
Nucleus - schoolphysics
... The explanation is that there is another force that acts only within the nucleus and between particles such as protons and neutrons. This is called the strong nuclear force. Within the nucleus this force is strong enough to overcome the electrostatic repulsion between the protons and so hold the nuc ...
... The explanation is that there is another force that acts only within the nucleus and between particles such as protons and neutrons. This is called the strong nuclear force. Within the nucleus this force is strong enough to overcome the electrostatic repulsion between the protons and so hold the nuc ...
Isotope

Isotopes are variants of a particular chemical element which differ in neutron number, although all isotopes of a given element have the same number of protons in each atom. The term isotope is formed from the Greek roots isos (ἴσος ""equal"") and topos (τόπος ""place""), meaning ""the same place""; thus, the meaning behind the name it is that different isotopes of a single element occupy the same position on the periodic table. The number of protons within the atom's nucleus is called atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number.For example, carbon-12, carbon-13 and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13 and 14 respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7 and 8 respectively.