atoms
... smaller and smaller particles – these are the atoms, and they still have properties of that element If you could line up 100,000,000 copper atoms in a single file, they would be approximately 1 cm long Despite their small size, individual atoms are observable with instruments such as scanning tunn ...
... smaller and smaller particles – these are the atoms, and they still have properties of that element If you could line up 100,000,000 copper atoms in a single file, they would be approximately 1 cm long Despite their small size, individual atoms are observable with instruments such as scanning tunn ...
Atoms, Isotopes, and Ions.pptx
... 3. How do we find the number of protons, neutrons, and electrons in an atom of an element? 4. What is the charge of protons, neutrons, and electrons? 5. Elements are are different because their atoms ...
... 3. How do we find the number of protons, neutrons, and electrons in an atom of an element? 4. What is the charge of protons, neutrons, and electrons? 5. Elements are are different because their atoms ...
Atomic Model Stations - Moore Public Schools
... A. _ _____________________________________________ B. _ ____________________________________________________ 7. Put 3 protons into the nucleus of the atom. Then fill in the following. Name of atom_______________________ atom or ion?______________ Net Charge_ ...
... A. _ _____________________________________________ B. _ ____________________________________________________ 7. Put 3 protons into the nucleus of the atom. Then fill in the following. Name of atom_______________________ atom or ion?______________ Net Charge_ ...
GEO143_lab_3_atoms_m..
... Sodium loses an electron to bond with chlorine. Does it become a positive or a negative ion? It becomes positive because it lost a negative charge, and is noted Na+. What happens to chlorine in order to bond to the sodium ion? Chlorine gains an electron, becoming a negative ion noted as Cl-. ...
... Sodium loses an electron to bond with chlorine. Does it become a positive or a negative ion? It becomes positive because it lost a negative charge, and is noted Na+. What happens to chlorine in order to bond to the sodium ion? Chlorine gains an electron, becoming a negative ion noted as Cl-. ...
Chapter 2 Atoms and Elements
... masses and abundances of isotopes are measured with a mass spectrometer atoms or molecules are ionized, then accelerated down a tube their path is bent by a magnetic field, separating them by mass ◦ similar to Thomson’s Cathode Ray ...
... masses and abundances of isotopes are measured with a mass spectrometer atoms or molecules are ionized, then accelerated down a tube their path is bent by a magnetic field, separating them by mass ◦ similar to Thomson’s Cathode Ray ...
Atomic Theory / Structure Powerpoint
... Earth - cool, heavy Water - wet Blend these in different proportions to get all substances ...
... Earth - cool, heavy Water - wet Blend these in different proportions to get all substances ...
Additional Topic 1 Atomic structure class booklet with syllabus and
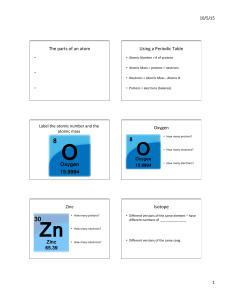
... PROTONS are POSITIVE and have a mass of 1 ATOMIC MASS UNIT (a.m.u). PROTONS are given the symbol p+ The NUMBER of PROTONS in the NUCLEUS of an atom give that atom its IDENTITY. The NUMBER of PROTONS in the NUCLEUS is given by the ATOMIC NUMBER. The atomic number for each element can be found ...
... PROTONS are POSITIVE and have a mass of 1 ATOMIC MASS UNIT (a.m.u). PROTONS are given the symbol p+ The NUMBER of PROTONS in the NUCLEUS of an atom give that atom its IDENTITY. The NUMBER of PROTONS in the NUCLEUS is given by the ATOMIC NUMBER. The atomic number for each element can be found ...
atomic I ppt R016solo2
... base was a mixture of radium salts and zinc sulfide. As the paint was mixed, the powdered base became airborne and drifted throughout the workroom causing the contents of the workroom, including the painters’ clothes and bodies, to glow in the dark. The paint is luminescent because radiation from th ...
... base was a mixture of radium salts and zinc sulfide. As the paint was mixed, the powdered base became airborne and drifted throughout the workroom causing the contents of the workroom, including the painters’ clothes and bodies, to glow in the dark. The paint is luminescent because radiation from th ...
Chapter 4 - Field Local Schools
... His ideas carried through middle ages. Alchemists change lead to gold ...
... His ideas carried through middle ages. Alchemists change lead to gold ...
Chapter 3 Nuclear Radiation
... In a balanced nuclear equation, the sum of the mass numbers and the sum of the atomic numbers are equal for the nuclei of the reactants and the ...
... In a balanced nuclear equation, the sum of the mass numbers and the sum of the atomic numbers are equal for the nuclei of the reactants and the ...
Sample pages 1 PDF
... (the drop method) – this of course also fixed the electron mass. The atomic nucleus Subsequently, different models of the atom were discussed, one of them being the model of Thomson. In this model, the electrons, and an equivalent number of positively charged particles are uniformly distributed thro ...
... (the drop method) – this of course also fixed the electron mass. The atomic nucleus Subsequently, different models of the atom were discussed, one of them being the model of Thomson. In this model, the electrons, and an equivalent number of positively charged particles are uniformly distributed thro ...
Symbols of Elements
... A sample of naturally occurring sulfur contains several isotopes with the following abundances Isotope % abundance 32S ...
... A sample of naturally occurring sulfur contains several isotopes with the following abundances Isotope % abundance 32S ...
Subatomic Particles
... Experiments using this setup were used to investigate the structure of atoms. Most of the particles traveled straight through the foil, but some alpha particles were deflected off to one side. Some were even deflected back toward the source. This was unexpected. Rutherford once said, “It was almost ...
... Experiments using this setup were used to investigate the structure of atoms. Most of the particles traveled straight through the foil, but some alpha particles were deflected off to one side. Some were even deflected back toward the source. This was unexpected. Rutherford once said, “It was almost ...
AtomMoleculeNaming_G1
... • The Observations That Led to an Atomic View of Matter • Dalton’s Atomic Theory • The Observations That Led to the Nuclear Atom Model • The Atomic Theory Today • Elements: A First Look at the Periodic Table • Compounds: Introduction to Bonding • Formulas, Names, and Masses of Compounds • Mixtures: ...
... • The Observations That Led to an Atomic View of Matter • Dalton’s Atomic Theory • The Observations That Led to the Nuclear Atom Model • The Atomic Theory Today • Elements: A First Look at the Periodic Table • Compounds: Introduction to Bonding • Formulas, Names, and Masses of Compounds • Mixtures: ...
chapter 4 presentation
... Teacher- summarized results of his experiments and those of others. Elements substances that can’t be broken down In Dalton’s Atomic Theory Combined idea of elements with that of atoms. ...
... Teacher- summarized results of his experiments and those of others. Elements substances that can’t be broken down In Dalton’s Atomic Theory Combined idea of elements with that of atoms. ...
Chapter 4 Atoms and Elements
... To determine the charge of each ion, use the ion charge equation.. Ion charge = #p – #e– The number of electrons is given in the problem. The number of protons is obtained from the element’s atomic number in the periodic table (a) magnesium with atomic number 12 Ion charge = 12 – 10 = 2+ (Mg2+) (b) ...
... To determine the charge of each ion, use the ion charge equation.. Ion charge = #p – #e– The number of electrons is given in the problem. The number of protons is obtained from the element’s atomic number in the periodic table (a) magnesium with atomic number 12 Ion charge = 12 – 10 = 2+ (Mg2+) (b) ...
Isotope

Isotopes are variants of a particular chemical element which differ in neutron number, although all isotopes of a given element have the same number of protons in each atom. The term isotope is formed from the Greek roots isos (ἴσος ""equal"") and topos (τόπος ""place""), meaning ""the same place""; thus, the meaning behind the name it is that different isotopes of a single element occupy the same position on the periodic table. The number of protons within the atom's nucleus is called atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number.For example, carbon-12, carbon-13 and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13 and 14 respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7 and 8 respectively.