Isotopes, Ions Worksheet
... b) Do different isotopes have different half-lifes (t ½ )? YES Different isotopes have a different neutron number which results in different half-life 21. List THREE Nuclear Applications 1. _______________________ 2. ______________________ 3. _______________________ 22. Why is it important to know t ...
... b) Do different isotopes have different half-lifes (t ½ )? YES Different isotopes have a different neutron number which results in different half-life 21. List THREE Nuclear Applications 1. _______________________ 2. ______________________ 3. _______________________ 22. Why is it important to know t ...
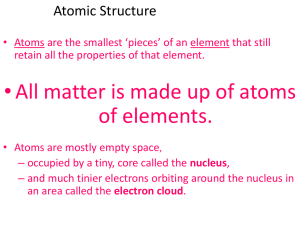
Atoms
... conferences or workshops. I am more than willing to share the presentation with anyone that contacts me at [email protected]. The images used in the presentation are not original and the presentation is distributed freely but only for classroom instruction. Rhonda Alexander ...
... conferences or workshops. I am more than willing to share the presentation with anyone that contacts me at [email protected]. The images used in the presentation are not original and the presentation is distributed freely but only for classroom instruction. Rhonda Alexander ...
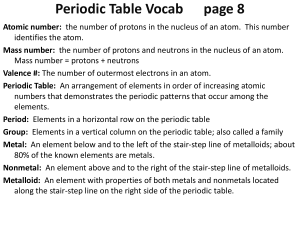
Periodic Table Vocab page 7
... Atomic number: the number of protons in the nucleus of an atom. This number identifies the atom. Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elem ...
... Atomic number: the number of protons in the nucleus of an atom. This number identifies the atom. Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elem ...
SNC 1D chem chpt2
... Each is 1840 times the mass of electrons. Neutrons have no charge Neutrons and protons are attracted through “strong force” attractions. Protons have a positive charge ...
... Each is 1840 times the mass of electrons. Neutrons have no charge Neutrons and protons are attracted through “strong force” attractions. Protons have a positive charge ...
2/1: Atomic Structure
... explosion has just started; surplus ships moored nearby can still be seen. ...
... explosion has just started; surplus ships moored nearby can still be seen. ...
Atomic Structure and Radioactivity
... Henri Becquerel found that uranium (U) exposed a photographic plate. In other words, uranium emitted penetrating radiation. ...
... Henri Becquerel found that uranium (U) exposed a photographic plate. In other words, uranium emitted penetrating radiation. ...
nuclear chemistry
... Nucleons can undergo 2 other types of decay o Positron emission ( same mass as an electron but positive charge) o Electron capture ( nuleus captures an electron from the electron cloud) NUCLEAR STABILITY Nuclei undergo decay to achieve stability If an isotopes mass number is greater than its a ...
... Nucleons can undergo 2 other types of decay o Positron emission ( same mass as an electron but positive charge) o Electron capture ( nuleus captures an electron from the electron cloud) NUCLEAR STABILITY Nuclei undergo decay to achieve stability If an isotopes mass number is greater than its a ...
Study Guide 1-3
... isotopic notation given a drawing of an atom or isotopic notation. You must also be able to determine number of protons, neutrons, and electrons present. A) ...
... isotopic notation given a drawing of an atom or isotopic notation. You must also be able to determine number of protons, neutrons, and electrons present. A) ...
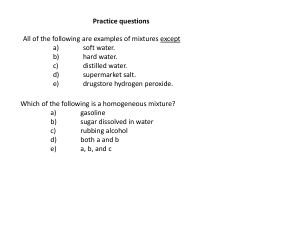
Practice questions
... K, Ag, Po, Cl, S b) P, Po, Ag, Cl, S c) Ph, K, Ag, S, Cl d) P, K, Ag, Cl, S e) Ph, Po, Ag, Cl, S ...
... K, Ag, Po, Cl, S b) P, Po, Ag, Cl, S c) Ph, K, Ag, S, Cl d) P, K, Ag, Cl, S e) Ph, Po, Ag, Cl, S ...
NUCLEAR CHEMISTRY
... •Any element with more than one proton (i.e., anything but hydrogen) will have repulsions between the protons in the nucleus. •A strong nuclear force helps keep the nucleus from flying apart. •Neutrons play a key role stabilizing the nucleus. •Therefore, the ratio of neutrons to protons is an import ...
... •Any element with more than one proton (i.e., anything but hydrogen) will have repulsions between the protons in the nucleus. •A strong nuclear force helps keep the nucleus from flying apart. •Neutrons play a key role stabilizing the nucleus. •Therefore, the ratio of neutrons to protons is an import ...
Periodic Table notes
... Things that occur in a repeating pattern on the periodic table Atomic number, # of energy levels, valence electrons, oxidation #, atomic size, metallic ...
... Things that occur in a repeating pattern on the periodic table Atomic number, # of energy levels, valence electrons, oxidation #, atomic size, metallic ...
Nuclear Reactions
... • Created by the spontaneous break down of a neutron to a proton and an electron. • Beta particles are faster and more penetrating than Alpha particles • Can be stopped by a sheet of aluminum ...
... • Created by the spontaneous break down of a neutron to a proton and an electron. • Beta particles are faster and more penetrating than Alpha particles • Can be stopped by a sheet of aluminum ...
Chapter 4 Atomic Structure Notes
... English physicist Sir James Chadwick - its mass nearly equals that of the proton C. Proton – was first observed in 1886 by E. Goldstein - A particle with one unit of a positive charge *** Atoms are electrically neutral*** ...
... English physicist Sir James Chadwick - its mass nearly equals that of the proton C. Proton – was first observed in 1886 by E. Goldstein - A particle with one unit of a positive charge *** Atoms are electrically neutral*** ...
Nuclear Chemistry
... Band of Stability • N/P ratio of stable nuclei • Stable small atoms (atomic # less than 20) are near 1/1 ratio • Stable large atoms are near 1.5/1 ratio. • Predict the stability of the following: carbon-12 mercury-200 hydrogen-3 uranium-238 ...
... Band of Stability • N/P ratio of stable nuclei • Stable small atoms (atomic # less than 20) are near 1/1 ratio • Stable large atoms are near 1.5/1 ratio. • Predict the stability of the following: carbon-12 mercury-200 hydrogen-3 uranium-238 ...
Dalton`s Atomic Theory
... Atoms have very small masses - the heaviest known atom is about 4 x 10−22 g Therefore, atomic mass unit (amu) is used to describe the mass of an individual atom The proton and neutron have nearly identical mass, and are much heavier than the electron (1 proton has same mass as 1836 electrons) ...
... Atoms have very small masses - the heaviest known atom is about 4 x 10−22 g Therefore, atomic mass unit (amu) is used to describe the mass of an individual atom The proton and neutron have nearly identical mass, and are much heavier than the electron (1 proton has same mass as 1836 electrons) ...
Structure of the Atom JJ Thomson- discovered the electron in late
... as protons are found to be at the center of this nucleus. James Chadwick- discovers the NEUTRON in 1932. The neutron is located in the nucleus and has NO CHARGE. The following table summarizes the subatomic particles listed in order of discovery: ...
... as protons are found to be at the center of this nucleus. James Chadwick- discovers the NEUTRON in 1932. The neutron is located in the nucleus and has NO CHARGE. The following table summarizes the subatomic particles listed in order of discovery: ...
Isotope

Isotopes are variants of a particular chemical element which differ in neutron number, although all isotopes of a given element have the same number of protons in each atom. The term isotope is formed from the Greek roots isos (ἴσος ""equal"") and topos (τόπος ""place""), meaning ""the same place""; thus, the meaning behind the name it is that different isotopes of a single element occupy the same position on the periodic table. The number of protons within the atom's nucleus is called atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number.For example, carbon-12, carbon-13 and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13 and 14 respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7 and 8 respectively.