Chemistry 199 - Oregon State chemistry
... We previously identified ammonia as a base because of its ability to accept a proton: :NH3 (aq) + H2O (l) H:NH3+ (aq) + OH- (aq) Ammonia is a Lewis base because it donates a pair of electrons to make a new bond (this pair of electrons is shown in the above reaction). Consider the reaction of a sil ...
... We previously identified ammonia as a base because of its ability to accept a proton: :NH3 (aq) + H2O (l) H:NH3+ (aq) + OH- (aq) Ammonia is a Lewis base because it donates a pair of electrons to make a new bond (this pair of electrons is shown in the above reaction). Consider the reaction of a sil ...
Chapter 21 Nuclear Chemistry
... 395,000 gallons of water in the core are heated to 619 F with a pressure of 2235 psi • Every 1.5 years 1/3 of the core is replaced. Takes 40 days to refuel – carried down man made “canals” to a huge swimming pool on site • Standing outside of a containment building you would get 5 milirem exposure o ...
... 395,000 gallons of water in the core are heated to 619 F with a pressure of 2235 psi • Every 1.5 years 1/3 of the core is replaced. Takes 40 days to refuel – carried down man made “canals” to a huge swimming pool on site • Standing outside of a containment building you would get 5 milirem exposure o ...
2007 - Physics Teacher
... A nuclear revival is possible. The global reserves of uranium could support a much larger number of reactors than exist today. Nuclear power generation could increase from three hundred gigawatts today to one thousand gigawatts by the year 2050, saving the earth from 1.5 billion tonnes of carbon emi ...
... A nuclear revival is possible. The global reserves of uranium could support a much larger number of reactors than exist today. Nuclear power generation could increase from three hundred gigawatts today to one thousand gigawatts by the year 2050, saving the earth from 1.5 billion tonnes of carbon emi ...
Page | 1 MATS1101 Chemistry notes semester 2 2012 TOPIC 1
... Using this theory we can explain three fundamental laws of chemical behaviour: 1. Law of Conservation of Mass and Energy: Matter is neither created or destroyed in a chemical reaction. Energy is neither created or destroyed in a chemical reaction, but it may be transformed from one form to another. ...
... Using this theory we can explain three fundamental laws of chemical behaviour: 1. Law of Conservation of Mass and Energy: Matter is neither created or destroyed in a chemical reaction. Energy is neither created or destroyed in a chemical reaction, but it may be transformed from one form to another. ...
Note 1.1 Chemistry of Life
... Atomic number is the number of protons found in the nucleus of the atom. It determines the particular atom identity. (Periodic Table) Atomic mass is the sum of the number of protons and neutrons found in the nucleus of an atom. Electrons are not found within the nucleus and do not contribute to the ...
... Atomic number is the number of protons found in the nucleus of the atom. It determines the particular atom identity. (Periodic Table) Atomic mass is the sum of the number of protons and neutrons found in the nucleus of an atom. Electrons are not found within the nucleus and do not contribute to the ...
Chemistry FINAL: CONTENT Review Packet
... _______________________is made from two or more substances that are physically combined The ability to do work is known as ________________ ________________________ are substances that are made up of only one type of atom ____________________________ is anything that has both mass and volume _______ ...
... _______________________is made from two or more substances that are physically combined The ability to do work is known as ________________ ________________________ are substances that are made up of only one type of atom ____________________________ is anything that has both mass and volume _______ ...
Notes matter energy
... number and type of atoms in a molecule. For example, H2SO4 (sulfuric acid) is the formula for a molecule because it consists of only nonmetals. The molecule is made up of 2 hydrogen atoms, 1 sulfur atom, and 4 oxygen atoms (and 7 total atoms). Subscripts indicate the number of atoms in the formula ( ...
... number and type of atoms in a molecule. For example, H2SO4 (sulfuric acid) is the formula for a molecule because it consists of only nonmetals. The molecule is made up of 2 hydrogen atoms, 1 sulfur atom, and 4 oxygen atoms (and 7 total atoms). Subscripts indicate the number of atoms in the formula ( ...
Name Date Class Period ______
... Name ______________________________________ Date __________________ Class Period _________ Atoms, Elements, and Compound Test Study Guide I. ...
... Name ______________________________________ Date __________________ Class Period _________ Atoms, Elements, and Compound Test Study Guide I. ...
Atoms, Ions and Molecules
... The number of neutrons does not change which element an atom belongs to. Atoms of the same element that have different numbers of neutrons are known as isotopes. Isotopes have idenBcal chemical properBes ...
... The number of neutrons does not change which element an atom belongs to. Atoms of the same element that have different numbers of neutrons are known as isotopes. Isotopes have idenBcal chemical properBes ...
Notes - Organization of Matter
... • Compounds are pure substances that are composed of two or more atoms that are chemically combined • Compounds can only be changed into simpler substances called elements by chemical changes ...
... • Compounds are pure substances that are composed of two or more atoms that are chemically combined • Compounds can only be changed into simpler substances called elements by chemical changes ...
Practice problems for chapter 1, 3 and 5 1) A small amount of salt
... 1) A small amount of salt dissolved in water is an example of a __________. 2) Which one of the following is a pure substance? A) concrete B) wood C) salt water D) elemental copper E) milk 3) For which of the following can the composition vary? A) pure substance B) element C) both homogeneous and he ...
... 1) A small amount of salt dissolved in water is an example of a __________. 2) Which one of the following is a pure substance? A) concrete B) wood C) salt water D) elemental copper E) milk 3) For which of the following can the composition vary? A) pure substance B) element C) both homogeneous and he ...
Matter
... nanometer is one billionth of a meter. Nanotechnology has shown that the behaviors and properties of some substances at the nanoscale contradict how they behave and what their properties are at the visible scale. Many products on the market today are already benefitting from nanotechnology such as: ...
... nanometer is one billionth of a meter. Nanotechnology has shown that the behaviors and properties of some substances at the nanoscale contradict how they behave and what their properties are at the visible scale. Many products on the market today are already benefitting from nanotechnology such as: ...
Study Island Copyright © 2012 Study Island
... 16. Hydrogen is a pure substance represented by the chemical symbol H. Which of the following best describes hydrogen? A. Hydrogen is an element made of two or more kinds of atoms. B. Hydrogen is an element made of only one kind of atom. C. Hydrogen is a compound made of only one kind of atom. D. Hy ...
... 16. Hydrogen is a pure substance represented by the chemical symbol H. Which of the following best describes hydrogen? A. Hydrogen is an element made of two or more kinds of atoms. B. Hydrogen is an element made of only one kind of atom. C. Hydrogen is a compound made of only one kind of atom. D. Hy ...
Practice problems for chapter 1, 2 and 3 1) A small amount of salt
... 1) A small amount of salt dissolved in water is an example of a __________. 2) Which one of the following is a pure substance? A) concrete B) wood C) salt water D) elemental copper E) milk 3) For which of the following can the composition vary? A) pure substance B) element C) both homogeneous and he ...
... 1) A small amount of salt dissolved in water is an example of a __________. 2) Which one of the following is a pure substance? A) concrete B) wood C) salt water D) elemental copper E) milk 3) For which of the following can the composition vary? A) pure substance B) element C) both homogeneous and he ...
Atomic Structure (history of atom)
... ATOMS of any one ELEMENT are different from those of any other element Atoms of different elements can physically mix together or chemically combine to form ...
... ATOMS of any one ELEMENT are different from those of any other element Atoms of different elements can physically mix together or chemically combine to form ...
PRACTICE PROBLEMS EXAM 1,2 and 3 1311
... 1) A small amount of salt dissolved in water is an example of a __________. 2) Which one of the following is a pure substance? A) concrete B) wood C) salt water D) elemental copper E) milk 3) For which of the following can the composition vary? A) pure substance B) element C) both homogeneous and he ...
... 1) A small amount of salt dissolved in water is an example of a __________. 2) Which one of the following is a pure substance? A) concrete B) wood C) salt water D) elemental copper E) milk 3) For which of the following can the composition vary? A) pure substance B) element C) both homogeneous and he ...
The Periodic Table HL Page 1 of 3 G. Galvin Name: Periodic Table
... -derive the electronic configurations of ions of s- and p-block elements only -describe the arrangement of electrons in individual orbitals of p-block atoms ...
... -derive the electronic configurations of ions of s- and p-block elements only -describe the arrangement of electrons in individual orbitals of p-block atoms ...
File - Mr. Walsh`s AP Chemistry
... For example, Co is the element cobalt, but CO is the compound carbon monoxide, which contains the elements carbon and oxygen. atomic number: the identity of an atom is based on the number of protons in its nucleus. (This works because the nucleus cannot be given to or shared with another atom.) The ...
... For example, Co is the element cobalt, but CO is the compound carbon monoxide, which contains the elements carbon and oxygen. atomic number: the identity of an atom is based on the number of protons in its nucleus. (This works because the nucleus cannot be given to or shared with another atom.) The ...
PHY140Y 33 Nuclear Properties - University of Toronto, Particle
... Lord Rutherford in 1909-1911 showed that the atom had to have a small, hard core that carried the bulk of the mass of the atom. Since atoms where neutral, the so-called nucleus of the atom had to have the same number of protons as the atom had electrons. This was the beginning of nuclear physics as ...
... Lord Rutherford in 1909-1911 showed that the atom had to have a small, hard core that carried the bulk of the mass of the atom. Since atoms where neutral, the so-called nucleus of the atom had to have the same number of protons as the atom had electrons. This was the beginning of nuclear physics as ...
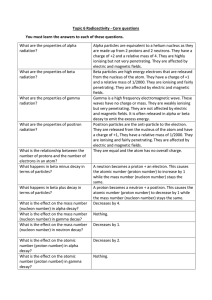
Topic 6 Radioactivity Core Questions
... Explain why ideas about the structure of the atom have changed over time. What is the difference between contamination and irradiation? ...
... Explain why ideas about the structure of the atom have changed over time. What is the difference between contamination and irradiation? ...
Dmitri Mendeleev
... The mass of an atom is measured relative to the mass of a chosen standard (carbon-12 atom), and is expressed in atomic mass units (amu). The average atomic mass of an element is the mass of that element’s natural occurring ...
... The mass of an atom is measured relative to the mass of a chosen standard (carbon-12 atom), and is expressed in atomic mass units (amu). The average atomic mass of an element is the mass of that element’s natural occurring ...
Fall Exam 1
... 13. This summer, a new mode of public transportation called the Hyperloop was proposed. The inventor claims the Hyperloop could reach average speeds of 598 miles per hour. Assuming that you could travel at this speed on the Hyperloop, how long would it take to go from Lexington to Chicago, IL (358.8 ...
... 13. This summer, a new mode of public transportation called the Hyperloop was proposed. The inventor claims the Hyperloop could reach average speeds of 598 miles per hour. Assuming that you could travel at this speed on the Hyperloop, how long would it take to go from Lexington to Chicago, IL (358.8 ...
Year 11 Chemistry Balancing Equations
... How do the number of protons, number of neutrons, and the mass number relate to each other? ...
... How do the number of protons, number of neutrons, and the mass number relate to each other? ...