Chemistry Final Review- Level 2
... _____ 1. Uranium forms thorium and helium, as shown in the equation below. ...
... _____ 1. Uranium forms thorium and helium, as shown in the equation below. ...
atom a very small particle that makes up most kinds of matters and
... two or more atoms of the same element that have different numbers of neutrons in the nuclei (same number of protons) states that mass is neither created nor destroyed and as a result the mass of the substances before a physical or chemical change is equal to the mass of the substances present after ...
... two or more atoms of the same element that have different numbers of neutrons in the nuclei (same number of protons) states that mass is neither created nor destroyed and as a result the mass of the substances before a physical or chemical change is equal to the mass of the substances present after ...
Classification of Matter
... Elements & Symbols • The symbol of an element is often taken from its name. • The first letter is always capitalized. • If an element starts with the same letter as another element, sometime the first two letters are used. • The second letter is always lowercase. • Some elements have symbols that d ...
... Elements & Symbols • The symbol of an element is often taken from its name. • The first letter is always capitalized. • If an element starts with the same letter as another element, sometime the first two letters are used. • The second letter is always lowercase. • Some elements have symbols that d ...
10) What do we call a highly ionized gas.
... 2) Inner electron shells are full. 3) 8 electrons present in the outermost (valence) shell. 4) Non-radioactive. ...
... 2) Inner electron shells are full. 3) 8 electrons present in the outermost (valence) shell. 4) Non-radioactive. ...
Prerequisite Knowledge for Chemistry
... A neutral atom of sodium would have 11 protons and 11 electrons. If a sodium atom were to lose a negatively charged electron as shown in the diagram the resulting atom would have a positive charge because it would have one more proton than electron. ...
... A neutral atom of sodium would have 11 protons and 11 electrons. If a sodium atom were to lose a negatively charged electron as shown in the diagram the resulting atom would have a positive charge because it would have one more proton than electron. ...
Chapter 10
... These molar ratios are used to 'convert' between any two compounds, whether they are reactants or products. This allows us to calculate moles of reactants needed, or products produced. ...
... These molar ratios are used to 'convert' between any two compounds, whether they are reactants or products. This allows us to calculate moles of reactants needed, or products produced. ...
Chemistry Review ATOMS
... • The mass of atoms and molecules is neither created nor destroyed in chemical reactions. – The # of atoms for each element in the reactants must equal the # of atoms for each element in the products in a chemical reaction. ...
... • The mass of atoms and molecules is neither created nor destroyed in chemical reactions. – The # of atoms for each element in the reactants must equal the # of atoms for each element in the products in a chemical reaction. ...
Unit 3 - Chemistry
... 1. All matter consists of ___________________. 2. Atoms of one element cannot be converted into atoms of another element. 3. Atoms of an element are identical in mass and other properties and are different from atoms of any other element. 4. _______________ result from the chemical combination of a ...
... 1. All matter consists of ___________________. 2. Atoms of one element cannot be converted into atoms of another element. 3. Atoms of an element are identical in mass and other properties and are different from atoms of any other element. 4. _______________ result from the chemical combination of a ...
Writing formulas and naming ionic bonds
... • The state of matter with a definite shape and definite volume • Solid • List the 3 types of nuclear radiation in order from most massive to least massive. • Alpha, beta, gamma ...
... • The state of matter with a definite shape and definite volume • Solid • List the 3 types of nuclear radiation in order from most massive to least massive. • Alpha, beta, gamma ...
Chapter 2
... • Fractional abundance: fraction of a total number of atoms, which consists of a particular isotope • Isotopic mass is not exactly equal to mass number – Neon-20, mass = 19.992 amu, abund = 0.9051 – Neon-21, mass = 20.994 amu, abund = 0.0027 – Neon-22, mass = 21.991 amu, abund = 0.0922 • Multiply is ...
... • Fractional abundance: fraction of a total number of atoms, which consists of a particular isotope • Isotopic mass is not exactly equal to mass number – Neon-20, mass = 19.992 amu, abund = 0.9051 – Neon-21, mass = 20.994 amu, abund = 0.0027 – Neon-22, mass = 21.991 amu, abund = 0.0922 • Multiply is ...
Physics, Chapter 45: Natural Radioactivity
... The discovery of an important phenomenon usually leads to other important discoveries. The discovery of x-rays by Roentgen in 1895 led to the discovery of radioactivity by Becquerel in 1896. In the gas type of x-ray tube used by Roentgen, the glass walls of the tube were observed to fluoresce. Becqu ...
... The discovery of an important phenomenon usually leads to other important discoveries. The discovery of x-rays by Roentgen in 1895 led to the discovery of radioactivity by Becquerel in 1896. In the gas type of x-ray tube used by Roentgen, the glass walls of the tube were observed to fluoresce. Becqu ...
Chemistry Part 1
... Matter—anything that occupies space and has mass (weight) Energy—the ability to do work ...
... Matter—anything that occupies space and has mass (weight) Energy—the ability to do work ...
Chapter 2 - profpaz.com
... (different mass numbers) and are called isotopes. Most elements have several isotopes, which are indicated by its chemical symbol, followed by a dash and the mass number of isotope. For example, the 3 isotopes of neon are shown below: ...
... (different mass numbers) and are called isotopes. Most elements have several isotopes, which are indicated by its chemical symbol, followed by a dash and the mass number of isotope. For example, the 3 isotopes of neon are shown below: ...
Unit 2 Lesson 3
... determined? • Radioactive isotopes, also called radioisotopes, are isotopes that are unstable and break down into other, stable isotopes by a process called radioactive decay. As they break down, they release excess energy by emitting radiation in the form of alpha, beta and gamma rays. • The radioa ...
... determined? • Radioactive isotopes, also called radioisotopes, are isotopes that are unstable and break down into other, stable isotopes by a process called radioactive decay. As they break down, they release excess energy by emitting radiation in the form of alpha, beta and gamma rays. • The radioa ...
Earth Materials
... -Diamond and graphite are both made of carbon (C), but why is one the hardest substance on Earth and the other very soft ? ...
... -Diamond and graphite are both made of carbon (C), but why is one the hardest substance on Earth and the other very soft ? ...
1 - WordPress.com
... mass of Z is 258.63 amu, which of these isotopes is most abundant? Since the atomic mass is the weighted average, the most abundant will be the isotope with the mass number closest to the atomic mass: Z-259 ...
... mass of Z is 258.63 amu, which of these isotopes is most abundant? Since the atomic mass is the weighted average, the most abundant will be the isotope with the mass number closest to the atomic mass: Z-259 ...
Appendix F - DigitalCommons@Olin
... engineer, and Ernest a lawyer. Ernest also played field hockey for Denmark at the 1948 Summer Olympics. ...
... engineer, and Ernest a lawyer. Ernest also played field hockey for Denmark at the 1948 Summer Olympics. ...
4.1Atoms and Isotopes
... abundant), C-13 (used in medical imagingMRI), and C-14 (used for dating fossils) Tin (Sn) has the most isotopes of any element at 10 Many isotopes are radioactive (unstable nucleus that will eventually break apart and release energy in sometimes harmful forms – eg. Gamma rays) Any isotope with an at ...
... abundant), C-13 (used in medical imagingMRI), and C-14 (used for dating fossils) Tin (Sn) has the most isotopes of any element at 10 Many isotopes are radioactive (unstable nucleus that will eventually break apart and release energy in sometimes harmful forms – eg. Gamma rays) Any isotope with an at ...
25.1 Nuclear Radiation
... somewhat harmful, gamma rays are particularly dangerous because they penetrate body tissues.) Explain that radioactivity reflects the tendency of atomic nuclei to achieve stability. Ask, What makes a nucleus unstable? (It has too many or too few neutrons relative to the number of protons.) Point out ...
... somewhat harmful, gamma rays are particularly dangerous because they penetrate body tissues.) Explain that radioactivity reflects the tendency of atomic nuclei to achieve stability. Ask, What makes a nucleus unstable? (It has too many or too few neutrons relative to the number of protons.) Point out ...
Slide 1
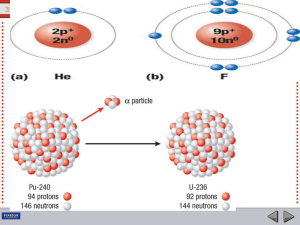
... When an atom ejects an alpha particle, the mass number of the resulting atom decreases by 4, and the atomic number by 2. The resulting atom belongs to an element two spaces back in the periodic table. When an atom ejects a beta particle from its nucleus, it loses no nucleons, its atomic number incre ...
... When an atom ejects an alpha particle, the mass number of the resulting atom decreases by 4, and the atomic number by 2. The resulting atom belongs to an element two spaces back in the periodic table. When an atom ejects a beta particle from its nucleus, it loses no nucleons, its atomic number incre ...
Dalton Model Reading
... chemical reactions is inconsistent with the existence of nuclear fusion and nuclear fission, but such processes are nuclear reactions and not chemical reactions. In addition, the idea that all atoms of a given element are identical in their physical and chemical properties is not precisely true, as ...
... chemical reactions is inconsistent with the existence of nuclear fusion and nuclear fission, but such processes are nuclear reactions and not chemical reactions. In addition, the idea that all atoms of a given element are identical in their physical and chemical properties is not precisely true, as ...
Notes 2 Balancing
... • The Law of Conservation of Mass • States that in ordinary chemical or physical changes, mass is neither created nor destroyed. • React vinegar and baking soda • Produces a gas (which “floats” away). • The products including this gas, if captured, is the same mass per mole as the reactants consumed ...
... • The Law of Conservation of Mass • States that in ordinary chemical or physical changes, mass is neither created nor destroyed. • React vinegar and baking soda • Produces a gas (which “floats” away). • The products including this gas, if captured, is the same mass per mole as the reactants consumed ...
Chemical Reactions & Balancing Equations
... – same number of atoms of each type of element on each side What if it isn’t balanced already? ...
... – same number of atoms of each type of element on each side What if it isn’t balanced already? ...
The Periodic table and subatomic particles
... 1. Read over notes in this package. 2. Redo these worksheets as well as extra practice sheets that have been provided. 3. Go through your grade 9 and 10 notes (should you still have them). 4. Topics to be covered include: Periodic table and its organization, subatomic particles, Bohr-Rutherford diag ...
... 1. Read over notes in this package. 2. Redo these worksheets as well as extra practice sheets that have been provided. 3. Go through your grade 9 and 10 notes (should you still have them). 4. Topics to be covered include: Periodic table and its organization, subatomic particles, Bohr-Rutherford diag ...