Please use your NUMERICAL RESPONSE SHEET to answer the
... Laura’s mom was busy preparing for your family’s Christmas feast. When she took out the silverware (that hadn’t been used since last Christmas!) she noticed that it was tarnished (turned black). Laura was given the job of polishing it up before everyone arrived – fun! As she was polishing it up, she ...
... Laura’s mom was busy preparing for your family’s Christmas feast. When she took out the silverware (that hadn’t been used since last Christmas!) she noticed that it was tarnished (turned black). Laura was given the job of polishing it up before everyone arrived – fun! As she was polishing it up, she ...
1. All matter is made up of
... 3. Use a magnet to separate them. 4. Pour the mixture into a filter. ...
... 3. Use a magnet to separate them. 4. Pour the mixture into a filter. ...
rp oc4
... 5. Circle the lone pair electrons in the following dot formula of water. 6. With respect to bonds formed between the following pairs of atoms: • Determine the electronegativity difference. SHOW WORK! • Determine the probable bond type (ionic, polar covalent, or nonpolar covalent). • Assign partial ...
... 5. Circle the lone pair electrons in the following dot formula of water. 6. With respect to bonds formed between the following pairs of atoms: • Determine the electronegativity difference. SHOW WORK! • Determine the probable bond type (ionic, polar covalent, or nonpolar covalent). • Assign partial ...
chemical reaction - Willmar Public Schools
... gold. They tried all sorts of chemical reactions involving lead, but they were never able to produce gold. Today, scientists know that one element cannot be changed into another by chemical processes. Radioactivity is the ability of an atom to emit, or give off, charged particles and energy from its ...
... gold. They tried all sorts of chemical reactions involving lead, but they were never able to produce gold. Today, scientists know that one element cannot be changed into another by chemical processes. Radioactivity is the ability of an atom to emit, or give off, charged particles and energy from its ...
AP Revision Guide Ch 18
... The alpha particles from any one type of decay all have the same energy, typically a few MeV. Being relatively massive charged particles, alpha particles ionise strongly. The range of alpha radiation in air is of the order a few centimetres. In solid materials, alpha particles are stopped very easil ...
... The alpha particles from any one type of decay all have the same energy, typically a few MeV. Being relatively massive charged particles, alpha particles ionise strongly. The range of alpha radiation in air is of the order a few centimetres. In solid materials, alpha particles are stopped very easil ...
Nuclear Physics
... A nucleon is a general term to denote a nuclear particle - that is, either a proton or a neutron. The atomic number Z of an element is equal to the number of protons in the nucleus of that element. The mass number A of an element is equal to the total number of nucleons (protons + neutrons). The mas ...
... A nucleon is a general term to denote a nuclear particle - that is, either a proton or a neutron. The atomic number Z of an element is equal to the number of protons in the nucleus of that element. The mass number A of an element is equal to the total number of nucleons (protons + neutrons). The mas ...
Variation in Properties of Group II Compounds
... Each group of elements embodied in the periodic table has their own unique properties. As for group II elements, they are classified as one of the s-block elements, also named as alkaline earth metals. In this essay, the variation in properties of group II elements and their compounds are illustrate ...
... Each group of elements embodied in the periodic table has their own unique properties. As for group II elements, they are classified as one of the s-block elements, also named as alkaline earth metals. In this essay, the variation in properties of group II elements and their compounds are illustrate ...
CHEM_Review - Kenston Local Schools
... Atoms that have the same number of protons and electrons are elect ically neutral. However, atoms may gain or lose electrons during chemical reactions. This creates an imbalance of negative and positive charges. Atoms may have a negative charge because they have gained extra electrons. Such atoms ar ...
... Atoms that have the same number of protons and electrons are elect ically neutral. However, atoms may gain or lose electrons during chemical reactions. This creates an imbalance of negative and positive charges. Atoms may have a negative charge because they have gained extra electrons. Such atoms ar ...
Chapter 2 - Chemistry
... John Dalton (British Chemist) - basic theory of modern chemistry - all matter whether element, compound or mixture is composed of small particles called atoms - purpose of atomic theory: to provide explanation of the structure of matter in terms of different combinations of very small particles ...
... John Dalton (British Chemist) - basic theory of modern chemistry - all matter whether element, compound or mixture is composed of small particles called atoms - purpose of atomic theory: to provide explanation of the structure of matter in terms of different combinations of very small particles ...
Element Symbol
... mixed and cannot be visibly distinguished. The particles of the substances are so small that they cannot be easily seen. 11. Another name for a homogeneous mixture is a solution. ...
... mixed and cannot be visibly distinguished. The particles of the substances are so small that they cannot be easily seen. 11. Another name for a homogeneous mixture is a solution. ...
4 Radioactive Elements
... valuable gold? More than a thousand years ago, many people thought it was a great idea, too. They tried everything they could think of. Of course, nothing worked. There is no chemical reaction that converts one element into another. Even so, elements do sometimes change into other elements. A uraniu ...
... valuable gold? More than a thousand years ago, many people thought it was a great idea, too. They tried everything they could think of. Of course, nothing worked. There is no chemical reaction that converts one element into another. Even so, elements do sometimes change into other elements. A uraniu ...
Document
... Aristotle disbelieved the ancient Greek theory of atoms being of different sizes, regular geometric shapes and being in constant motion. He didn't think atoms could be in constant motion in an empty space. Aristotle’s theory was used for almost 2000 years, until after the scientific revolution, when ...
... Aristotle disbelieved the ancient Greek theory of atoms being of different sizes, regular geometric shapes and being in constant motion. He didn't think atoms could be in constant motion in an empty space. Aristotle’s theory was used for almost 2000 years, until after the scientific revolution, when ...
CHAPTER 2: ATOMS, IONS, AND COMPOUNDS
... Empedocles (490-430 B.C.): suggested all matter was composed of four basic elements: air, water, fire, and earth. Aristotle (384-321 B.C.): accepted Empedocles idea and added a fifth element, heavenly ether, which is perfect, eternal, and incorruptible. Aristotle’s idea of five basic elements was ac ...
... Empedocles (490-430 B.C.): suggested all matter was composed of four basic elements: air, water, fire, and earth. Aristotle (384-321 B.C.): accepted Empedocles idea and added a fifth element, heavenly ether, which is perfect, eternal, and incorruptible. Aristotle’s idea of five basic elements was ac ...
Unit 2 Review Questions Fill in the blank In a(n) change, a new
... is a mixture of metals. c. A solid produced when two solutions are mixed together is a(n) ...
... is a mixture of metals. c. A solid produced when two solutions are mixed together is a(n) ...
Modern Physics MC Practice Answers
... X–rays do not have a charge so would not be deflected by a magnetic field. All of the rest of the listed properties are true however. a) x rays clearly pass through light materials as evidenced from their use in the medical field. b) From Bohr’s energy level diagram for hydrogren we can conclude thi ...
... X–rays do not have a charge so would not be deflected by a magnetic field. All of the rest of the listed properties are true however. a) x rays clearly pass through light materials as evidenced from their use in the medical field. b) From Bohr’s energy level diagram for hydrogren we can conclude thi ...
Covalent Bonds - WordPress.com
... • A molecule consists of two or more atoms held together by covalent bonds • A single covalent bond, or single bond, is the sharing of only one pair of valence electrons • A double covalent bond, or double bond, is the sharing of two pairs of valence electrons • The double bonds are stronger than s ...
... • A molecule consists of two or more atoms held together by covalent bonds • A single covalent bond, or single bond, is the sharing of only one pair of valence electrons • A double covalent bond, or double bond, is the sharing of two pairs of valence electrons • The double bonds are stronger than s ...
Chapter 16 Nuclear Chemistry - An Introduction to Chemistry
... stability in one of two ways, positron emission or electron capture. In positron emission (), a proton becomes a neutron and a positron. The neutron stays in the nucleus, and the positron speeds out of the nucleus at high velocity. 24. Because radioactive decay leads to more stable products, it al ...
... stability in one of two ways, positron emission or electron capture. In positron emission (), a proton becomes a neutron and a positron. The neutron stays in the nucleus, and the positron speeds out of the nucleus at high velocity. 24. Because radioactive decay leads to more stable products, it al ...
Chemistry
... taking into account the percent and mass of each different isotope. C4.10e C4.10c Calculate the average atomic mass of an element given the percent abundance and mass of the individual isotopes. C4.10d Predict which isotope will have the greatest abundance given the possible isotopes for an element ...
... taking into account the percent and mass of each different isotope. C4.10e C4.10c Calculate the average atomic mass of an element given the percent abundance and mass of the individual isotopes. C4.10d Predict which isotope will have the greatest abundance given the possible isotopes for an element ...
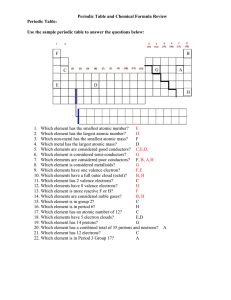
Periodic Table Review Key
... Would atom A gain or lose valence electrons? __lose__ Would atom B gain or lose valence electrons? __neither__ ...
... Would atom A gain or lose valence electrons? __lose__ Would atom B gain or lose valence electrons? __neither__ ...
Unit 2 Review for Test
... 40. What elements make up a protein? 42. Name the building blocks of lipids. 43. Draw a structural diagram showing a simple representation of a fatty acid.. 44. List some types of lipids. 45. Name the primary use of the type of macromolecule which is a source of energy. 46. Name the macromolecule wh ...
... 40. What elements make up a protein? 42. Name the building blocks of lipids. 43. Draw a structural diagram showing a simple representation of a fatty acid.. 44. List some types of lipids. 45. Name the primary use of the type of macromolecule which is a source of energy. 46. Name the macromolecule wh ...
Atomic Theories- Part I - Tenafly Public Schools
... The word atom comes from the Greek and means “indivisible”. ...
... The word atom comes from the Greek and means “indivisible”. ...
Atoms and Spectral Lines
... • Chemical element: Defined by the number of protons in the atomic nucleus ("atomic number") • Isotope: Each element can have different isotopes, defined by number of neutrons. – Only a few isotopes of each element are stable (the others are radioactive and come apart quickly): – there are typically ...
... • Chemical element: Defined by the number of protons in the atomic nucleus ("atomic number") • Isotope: Each element can have different isotopes, defined by number of neutrons. – Only a few isotopes of each element are stable (the others are radioactive and come apart quickly): – there are typically ...
Topic 7_2__Radioactive decay
... The gamma rays are uncharged and very high energy. They can travel a few centimeters in lead, or a very ...
... The gamma rays are uncharged and very high energy. They can travel a few centimeters in lead, or a very ...