7.2- Nuclear reactions (PPT)
... ▪ From his experience with alpha emitters, Rutherford thought that alpha ...
... ▪ From his experience with alpha emitters, Rutherford thought that alpha ...
Presentation
... present in any amount. S u b s t a n c e s c a n b e separated by simple p h y s i c a l m e a n s. ...
... present in any amount. S u b s t a n c e s c a n b e separated by simple p h y s i c a l m e a n s. ...
Science Outline NHPS: Chemistry
... different questions. D INQ.5 Identify independent and dependent variables, including those that are kept constant and those used as controls. D INQ.6 Use appropriate tools and techniques to ...
... different questions. D INQ.5 Identify independent and dependent variables, including those that are kept constant and those used as controls. D INQ.6 Use appropriate tools and techniques to ...
High School Curriculum Standards: Chemistry
... Chemistry is the study of matter—its properties and its changes. The idea that matter is made up of particles is over 2000 years old, but the idea of using properties of these particles to explain observable characteristics of matter has more recent origins. In ancient Greece, it was proposed that m ...
... Chemistry is the study of matter—its properties and its changes. The idea that matter is made up of particles is over 2000 years old, but the idea of using properties of these particles to explain observable characteristics of matter has more recent origins. In ancient Greece, it was proposed that m ...
File
... _____ 14. The activity series of metals can be used to predict products in double-replacement reactions. _____ 15. Carbon dioxide and water are the products of the combustion of hexane (C6H14). _____ 16. A nonmetal can replace another nonmetal from a compound in a single-replacement reaction. ...
... _____ 14. The activity series of metals can be used to predict products in double-replacement reactions. _____ 15. Carbon dioxide and water are the products of the combustion of hexane (C6H14). _____ 16. A nonmetal can replace another nonmetal from a compound in a single-replacement reaction. ...
Chapter 8
... Electron affinity is the negative of the energy change that occurs when an electron is accepted by an atom in the gaseous state to form an anion. X (g) + e- ...
... Electron affinity is the negative of the energy change that occurs when an electron is accepted by an atom in the gaseous state to form an anion. X (g) + e- ...
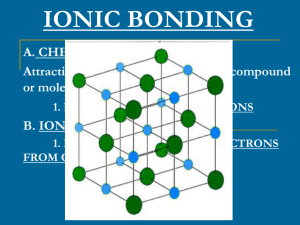
ionic bonding - Old Saybrook Public Schools
... 3. While metal atoms are usually larger than nonmetal atoms in the same period, POSITIVE ions are usually SMALLER than negative ions (of the same period). 4. Periodic trends in ion size Compare the sizes of the ions of Mg , Al , Na Na+ Mg2+ Al3+ Of the ions of P , S , and Cl P3- S2- Cl 1inc ...
... 3. While metal atoms are usually larger than nonmetal atoms in the same period, POSITIVE ions are usually SMALLER than negative ions (of the same period). 4. Periodic trends in ion size Compare the sizes of the ions of Mg , Al , Na Na+ Mg2+ Al3+ Of the ions of P , S , and Cl P3- S2- Cl 1inc ...
High School Chemistry
... f. After researching, evaluate and report the effects of nuclear radiation on humans or other organisms. Science language students should use: Atom, element, nucleus, proton, neutron, electron, metalloid, periodic table, isotope, metal, half-life, fission, fusion, nonmetal, quanta, photon, waveleng ...
... f. After researching, evaluate and report the effects of nuclear radiation on humans or other organisms. Science language students should use: Atom, element, nucleus, proton, neutron, electron, metalloid, periodic table, isotope, metal, half-life, fission, fusion, nonmetal, quanta, photon, waveleng ...
QUIZ: History of Atomic Structure
... B) all forms of matter contain electrons C) all positive rays were actually protons D) all alpha particles are heavier than protons 3. Many classic experiments have given us indirect evidence of the nature of the atom. Which of the experiments listed below did not give the results described? A) The ...
... B) all forms of matter contain electrons C) all positive rays were actually protons D) all alpha particles are heavier than protons 3. Many classic experiments have given us indirect evidence of the nature of the atom. Which of the experiments listed below did not give the results described? A) The ...
Unit 2 Part I PowerPoint
... specify a particular isotope of the element and provide other important information. The atomic number is written as a subscript on the left of the element symbol, the mass number is written as a superscript on the left of the element symbol • Mass number - The total number of protons and neutrons i ...
... specify a particular isotope of the element and provide other important information. The atomic number is written as a subscript on the left of the element symbol, the mass number is written as a superscript on the left of the element symbol • Mass number - The total number of protons and neutrons i ...
Presentation
... that can be broken down by chemical methods When they are broken down, the pieces have completely different properties than the compound. Made of molecules- two or more atoms ...
... that can be broken down by chemical methods When they are broken down, the pieces have completely different properties than the compound. Made of molecules- two or more atoms ...
Unit 2 Spiraling
... one position and vibrates around that position. This results in a definite shape and volume. The particles in a liquid stay relatively close together, but they can move around each other (rotate or tumble). This results in a definite volume but no definite shape. Gas particles are far apart; they mo ...
... one position and vibrates around that position. This results in a definite shape and volume. The particles in a liquid stay relatively close together, but they can move around each other (rotate or tumble). This results in a definite volume but no definite shape. Gas particles are far apart; they mo ...
Elements, Compounds and Chemical Reactions
... An atom is the basic unit of an element. The Periodic Table lists all the known elements ...
... An atom is the basic unit of an element. The Periodic Table lists all the known elements ...
1 - Groupfusion.net
... mass of Z is 258.63 amu, which of these isotopes is most abundant? Since the atomic mass is the weighted average, the most abundant will be the isotope with the mass number closest to the atomic mass: Z-259 ...
... mass of Z is 258.63 amu, which of these isotopes is most abundant? Since the atomic mass is the weighted average, the most abundant will be the isotope with the mass number closest to the atomic mass: Z-259 ...
Matter and Energy
... -determines the number of molecules (groups) of the formula -This number will be DISTRIBUTED just like in math. It applies to each element and is multiplied by each subscript to find the total number of atoms of each element and a total number of atoms in the molecule. ...
... -determines the number of molecules (groups) of the formula -This number will be DISTRIBUTED just like in math. It applies to each element and is multiplied by each subscript to find the total number of atoms of each element and a total number of atoms in the molecule. ...
Family
... Professor Dmitri Mendeleev published the first Periodic Table of the Atomic Elements in 1869 based on properties which appeared with some regularity as he laid out the elements from lightest to heaviest. When Mendeleev proposed his periodic table, he noted gaps in the table, and predicted that as-of ...
... Professor Dmitri Mendeleev published the first Periodic Table of the Atomic Elements in 1869 based on properties which appeared with some regularity as he laid out the elements from lightest to heaviest. When Mendeleev proposed his periodic table, he noted gaps in the table, and predicted that as-of ...
p Atomic Structure notes packet 14_15
... Source of energy in the core of the Earth that produces heat from the decay of radioactive elements. Produces vast quantities of energy. Does not produce particulate air pollution like fossil fuels and coal. Involves the splitting of harmful radioactive elements. Loss of control leads to harmful rad ...
... Source of energy in the core of the Earth that produces heat from the decay of radioactive elements. Produces vast quantities of energy. Does not produce particulate air pollution like fossil fuels and coal. Involves the splitting of harmful radioactive elements. Loss of control leads to harmful rad ...
Lecture 16: Iron Core Collapse, Neutron Stars, and Nucleosynthesis
... of the supernova, matter is abruptly raised to a higher temperature. Since nuclear reactions occur at rates that are very sensitive to the temperature, this causes an increase in the burning. New elements are created in seconds that it might otherwise have taken weeks and months to synthesize. ...
... of the supernova, matter is abruptly raised to a higher temperature. Since nuclear reactions occur at rates that are very sensitive to the temperature, this causes an increase in the burning. New elements are created in seconds that it might otherwise have taken weeks and months to synthesize. ...
Chapter 2 (Hill/Petrucci/McCreary/Perry This chapter deals with
... Dalton's atomic theory had several postulates: 1. all matter is composed of small, invisible particles called atoms 2. in chemical reactions, atoms are neither created nor destroyed 3. atoms of each element have unique properties - all atoms of a given atom are identical and have identical masses an ...
... Dalton's atomic theory had several postulates: 1. all matter is composed of small, invisible particles called atoms 2. in chemical reactions, atoms are neither created nor destroyed 3. atoms of each element have unique properties - all atoms of a given atom are identical and have identical masses an ...
Chemistry EOC Review 2015 Name Per ___ This review is part of
... Ionization energy is the energy required to remove an electron from an atom or ion. The smaller the atom, the closer the valence electrons are to the nucleus and therefore, the tighter the electrons are being held. This gives the smallest atoms the largest ionization energy. As you go down a family/ ...
... Ionization energy is the energy required to remove an electron from an atom or ion. The smaller the atom, the closer the valence electrons are to the nucleus and therefore, the tighter the electrons are being held. This gives the smallest atoms the largest ionization energy. As you go down a family/ ...
Document
... Matter is anything that has mass and occupies space. All matter is made of atoms. Atoms are the smallest form of elements. About 100 elements • Hydrogen is an element that accounts for about 90% of total mass of the universe. • Hydrogen makes up about 1% of Earth’s crust and most of that is in wate ...
... Matter is anything that has mass and occupies space. All matter is made of atoms. Atoms are the smallest form of elements. About 100 elements • Hydrogen is an element that accounts for about 90% of total mass of the universe. • Hydrogen makes up about 1% of Earth’s crust and most of that is in wate ...
- Aboriginal Access to Engineering
... white willow bark called salicin. Aspirin is produced in a complex industrial chemical process and reduces fever, swelling and pain. Willow bark tea does exactly the same thing because boiling the bark in water releases salicin. The healing properties of willow bark have been known to the Chinese an ...
... white willow bark called salicin. Aspirin is produced in a complex industrial chemical process and reduces fever, swelling and pain. Willow bark tea does exactly the same thing because boiling the bark in water releases salicin. The healing properties of willow bark have been known to the Chinese an ...
Chemistry Reference Table Review
... 83. What are two properties of most nonmetals? (1) high ionization energy and poor electrical conductivity (2) high ionization energy and good electrical conductivity (3) low ionization energy and poor electrical conductivity (4) low ionization energy and good electrical conductivity 84. Based on Ta ...
... 83. What are two properties of most nonmetals? (1) high ionization energy and poor electrical conductivity (2) high ionization energy and good electrical conductivity (3) low ionization energy and poor electrical conductivity (4) low ionization energy and good electrical conductivity 84. Based on Ta ...
CHAPTER 2 The nucleus and radioactive decay - Cin
... binding energy is fundamental to understanding why certain elements undergo radioactive decay. A nuclide will energetically tend towards decay by a particular mode (α emission, β emission, or spontaneous fission) if its atomic mass is greater than the sum of the masses of the products formed by that ...
... binding energy is fundamental to understanding why certain elements undergo radioactive decay. A nuclide will energetically tend towards decay by a particular mode (α emission, β emission, or spontaneous fission) if its atomic mass is greater than the sum of the masses of the products formed by that ...