Exercises 2013 - Oxford School on Neutron Scattering
... A neutron of spin ½ interacts with a nucleus of spin I to form two states in which the spins are either parallel or antiparallel. The combined spin J of these states is J = I + ½ and J = I – ½, respectively. Different scattering lengths (amplitudes) b+, b– are associated with these states. The proba ...
... A neutron of spin ½ interacts with a nucleus of spin I to form two states in which the spins are either parallel or antiparallel. The combined spin J of these states is J = I + ½ and J = I – ½, respectively. Different scattering lengths (amplitudes) b+, b– are associated with these states. The proba ...
Chapter 5 - apel slice
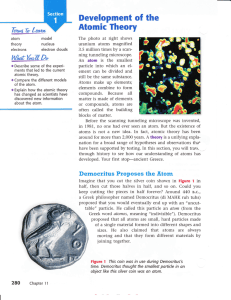
... The search for a description of matter began with the Greek philosopher Democritus (dihMAHK-ruh-tuhs) more than 2000 years ago. He and many other philosophers had puzzled over this question: Could matter be divided into smaller and smaller pieces forever, or was there a limit to the number of times ...
... The search for a description of matter began with the Greek philosopher Democritus (dihMAHK-ruh-tuhs) more than 2000 years ago. He and many other philosophers had puzzled over this question: Could matter be divided into smaller and smaller pieces forever, or was there a limit to the number of times ...
URL - StealthSkater
... Nuclear Force” of a stable isotope of Element-115 was used to provide the Sport Model Flying Disc gravity field propulsion system may very well be true. But one problem! There are no known stable Elements above Element-83 Bismuth. Physicists have only recently produced 2 isotopes of Element115 in a ...
... Nuclear Force” of a stable isotope of Element-115 was used to provide the Sport Model Flying Disc gravity field propulsion system may very well be true. But one problem! There are no known stable Elements above Element-83 Bismuth. Physicists have only recently produced 2 isotopes of Element115 in a ...
Band structure effects for dripped neutrons in neutron star crust
... multiplication) of the momentum k (related to the translational symmetry), each sheet in momentum space being specified by a band index α (associated with rotational symmetry). Recently Magierski et al. [6] have suggested that shell effects arising from “unbound” neutrons may be important for neutro ...
... multiplication) of the momentum k (related to the translational symmetry), each sheet in momentum space being specified by a band index α (associated with rotational symmetry). Recently Magierski et al. [6] have suggested that shell effects arising from “unbound” neutrons may be important for neutro ...
Chem101 - Lecture 2 Elements Elements as Pure
... determined by comparing them to the mass of the carbon-12 isotope. • The unit of mass that is used is called the atomic mass unit and is represented by the symbol u. • The atomic mass unit is equal to exactly 1/12 the mass of the carbon-12 ...
... determined by comparing them to the mass of the carbon-12 isotope. • The unit of mass that is used is called the atomic mass unit and is represented by the symbol u. • The atomic mass unit is equal to exactly 1/12 the mass of the carbon-12 ...
Build an Atom Scripted - UTeach Outreach
... Since all carbon atoms have six protons, an atom of the carbon isotope with six neutrons has a mass number of 12, and an atom of the carbon isotope with seven neutrons has a mass number of 13. In order to determine the mass of a mixed quantity of carbon atoms, we take a weighted average of the masse ...
... Since all carbon atoms have six protons, an atom of the carbon isotope with six neutrons has a mass number of 12, and an atom of the carbon isotope with seven neutrons has a mass number of 13. In order to determine the mass of a mixed quantity of carbon atoms, we take a weighted average of the masse ...
Build an Atom Scripted
... Since all carbon atoms have six protons, an atom of the carbon isotope with six neutrons has a mass number of 12, and an atom of the carbon isotope with seven neutrons has a mass number of 13. In order to determine the mass of a mixed quantity of carbon atoms, we take a weighted average of the masse ...
... Since all carbon atoms have six protons, an atom of the carbon isotope with six neutrons has a mass number of 12, and an atom of the carbon isotope with seven neutrons has a mass number of 13. In order to determine the mass of a mixed quantity of carbon atoms, we take a weighted average of the masse ...
File
... Who was the man who lived from 460B.C.–370B.C. and was among the first to suggest the idea of atoms? a. Atomos c. Democritus b. Dalton d. Thomson The smallest particle of an element that retains the properties of that element is a(n) ____. a. atom c. proton b. electron d. neutron Which of the follow ...
... Who was the man who lived from 460B.C.–370B.C. and was among the first to suggest the idea of atoms? a. Atomos c. Democritus b. Dalton d. Thomson The smallest particle of an element that retains the properties of that element is a(n) ____. a. atom c. proton b. electron d. neutron Which of the follow ...
Atoms
... • 4. Which of these is a correct definition of an isotope? – a. different versions of an element that have a different number of neutrons – b. atoms of the same element with the same atomic number but different mass number – c. different version of an element that have the different number of electr ...
... • 4. Which of these is a correct definition of an isotope? – a. different versions of an element that have a different number of neutrons – b. atoms of the same element with the same atomic number but different mass number – c. different version of an element that have the different number of electr ...
Nuclear Chemistry - Somerset Academy
... • Note that the initial amount may be in units of mass or number of particles. • A more versatile form of the equation can be written if the exponent n is replaced by the equivalent quantity t/T, where t is the elapsed time and T is the duration of the half-life. ...
... • Note that the initial amount may be in units of mass or number of particles. • A more versatile form of the equation can be written if the exponent n is replaced by the equivalent quantity t/T, where t is the elapsed time and T is the duration of the half-life. ...
chem pre ap atom and nuclear practice test
... c. of each element are identical in size, mass, and other properties. d. of different elements cannot combine. 2. Which of the following statements is true? a. Atoms of the same element may have different masses. b. Atoms may be divided in ordinary chemical reactions. c. Atoms can never combine with ...
... c. of each element are identical in size, mass, and other properties. d. of different elements cannot combine. 2. Which of the following statements is true? a. Atoms of the same element may have different masses. b. Atoms may be divided in ordinary chemical reactions. c. Atoms can never combine with ...
Chemistry Lecture No.4______By : Asst. Lect. Tariq-H-AL
... these ionization reactions. Repeated exposure to low levels of radiation seems to have a number of major effects on health. Among them are cancer (carcinogenic effects), damage to the fetus, and genetic damage. It has been known for many years that radiation causes cancer. Skin cancer, bone cancer, ...
... these ionization reactions. Repeated exposure to low levels of radiation seems to have a number of major effects on health. Among them are cancer (carcinogenic effects), damage to the fetus, and genetic damage. It has been known for many years that radiation causes cancer. Skin cancer, bone cancer, ...
Week 13 Chemistry
... massive than alpha particles. Beta particles are positively charged and more massive than alpha particles. Beta particles are negatively charged and more massive than alpha particles. ...
... massive than alpha particles. Beta particles are positively charged and more massive than alpha particles. Beta particles are negatively charged and more massive than alpha particles. ...
Development of the Atomic Theory
... learned how the atomic theory developed through centuries of observation and experimentation. Now it's time to learn about the atom itself. In this section, you'll learn about the particles inside the atom, and you'll learn about the forces that act on those particles. But first you'll find out just ...
... learned how the atomic theory developed through centuries of observation and experimentation. Now it's time to learn about the atom itself. In this section, you'll learn about the particles inside the atom, and you'll learn about the forces that act on those particles. But first you'll find out just ...
The Structure of the Atom 4
... 69.723 amu, has two naturally occurring isotopes, Ga-69 and Ga-71. Which isotope occurs in greater abundance? Explain. ...
... 69.723 amu, has two naturally occurring isotopes, Ga-69 and Ga-71. Which isotope occurs in greater abundance? Explain. ...
Journal of Theoretics MODELS OF THE ATOMIC NUCLEI
... The analysis of the laws of formation of the spectra shows that the electrons in the atoms do not have an orbital movement. The electron interacts with the nucleus as a bar magnet, i.e. with its axis of rotation. The unlike electric fields of the electron and the proton draw them together, and the l ...
... The analysis of the laws of formation of the spectra shows that the electrons in the atoms do not have an orbital movement. The electron interacts with the nucleus as a bar magnet, i.e. with its axis of rotation. The unlike electric fields of the electron and the proton draw them together, and the l ...
Chapter 17 Resource: Properties of Atoms and the Periodic Table
... Directions: Use the terms below to complete the following paragraphs about atoms , atomic mass, and isotopes. Terms may be used more than once. six number electrons isotopes electron cloud neutron(s) proton(s) mass quarks six protons The electron has very little mass compared to the 1. _____________ ...
... Directions: Use the terms below to complete the following paragraphs about atoms , atomic mass, and isotopes. Terms may be used more than once. six number electrons isotopes electron cloud neutron(s) proton(s) mass quarks six protons The electron has very little mass compared to the 1. _____________ ...
Atoms, Isotopes and Relative Atomic Masses MS
... (the mass of a) carbon-12 OR 12C (atom) IGNORE Reference to average OR weighted mean (i.e. correct definition of relative atomic mass will score both marks) ALLOW mass of a mole of the isotope/atom with 1/12th the mass of a mole OR 12 g of carbon-12 for two marks. ALLOW 2 marks for: ‘Mass of the iso ...
... (the mass of a) carbon-12 OR 12C (atom) IGNORE Reference to average OR weighted mean (i.e. correct definition of relative atomic mass will score both marks) ALLOW mass of a mole of the isotope/atom with 1/12th the mass of a mole OR 12 g of carbon-12 for two marks. ALLOW 2 marks for: ‘Mass of the iso ...
fractal physics theory - nucleons and the strong force
... over the cs-neutron’s surface so an approximation is made by calculating the E at the center of five slab areas. In Figure 3 below, the five blue circles drawn beyond the csproton’s surface represent five electric equipotential surfaces all centered on the origin of the axes, with radii as listed. T ...
... over the cs-neutron’s surface so an approximation is made by calculating the E at the center of five slab areas. In Figure 3 below, the five blue circles drawn beyond the csproton’s surface represent five electric equipotential surfaces all centered on the origin of the axes, with radii as listed. T ...
atom
... 3- Atoms of different elements can combine in simple whole number ratios to form compounds. 4 – Chemical reactions occur when atoms are separated, joined or rearranged. However, atoms of one element are not changed into atoms of another element by a chemical reaction. NC Competency Goal 2 ...
... 3- Atoms of different elements can combine in simple whole number ratios to form compounds. 4 – Chemical reactions occur when atoms are separated, joined or rearranged. However, atoms of one element are not changed into atoms of another element by a chemical reaction. NC Competency Goal 2 ...
Introduction to Atomic Structure - New Jersey Center for Teaching
... does change, and atoms are changed from one type to another. You learned about these last year in Physics. They are called Nuclear Reactions. Also remember that today we know atoms can be broken down into smaller bits. We also know all atoms of an element are not identical elements found in nature c ...
... does change, and atoms are changed from one type to another. You learned about these last year in Physics. They are called Nuclear Reactions. Also remember that today we know atoms can be broken down into smaller bits. We also know all atoms of an element are not identical elements found in nature c ...
Introduction to Atomic Structure - New Jersey Center for Teaching
... The anode rays were referred to as protons, which were found to be significantly heavier than electrons. 1 proton = 1840 x mass of electron Since the heaviest anode rays in oxygen were found to be 8 x heavier than those in hydrogen, it was assumed that oxygen had 8 protons compared to hydrogen's 1. ...
... The anode rays were referred to as protons, which were found to be significantly heavier than electrons. 1 proton = 1840 x mass of electron Since the heaviest anode rays in oxygen were found to be 8 x heavier than those in hydrogen, it was assumed that oxygen had 8 protons compared to hydrogen's 1. ...
Problems - El Camino College
... c) The mass or an electron 1s about the same as the mass ofa proton. d) There are subatom ic particles in addition to the electron, proton. and neutron. e) The mass of an atom i~ uniformly distributed throughout the atom. f) Most of the particles fucd i11to the gold foi l in the Rutherford experimen ...
... c) The mass or an electron 1s about the same as the mass ofa proton. d) There are subatom ic particles in addition to the electron, proton. and neutron. e) The mass of an atom i~ uniformly distributed throughout the atom. f) Most of the particles fucd i11to the gold foi l in the Rutherford experimen ...
CHAPTER 1 INTRODUCTION 1.1 Preview
... second which would yield a low intensity thermal neutron beam after moderation and collimation. ...
... second which would yield a low intensity thermal neutron beam after moderation and collimation. ...