PART I TORT LIABILITY AND RADIATION INJURIES
... additional proton in the nucleus changes the atom to an element one higher on the atomic scale. Where two or more neutrons turn into protons, several transmutations may occur. This process is spoken of as beta decay. Beta radiation is the most common mode of decay of radioisotopes. At this point it ...
... additional proton in the nucleus changes the atom to an element one higher on the atomic scale. Where two or more neutrons turn into protons, several transmutations may occur. This process is spoken of as beta decay. Beta radiation is the most common mode of decay of radioisotopes. At this point it ...
View PDF
... Rutherford fired positively charged particles at metal foil and concluded that most of the mass of an atom was a. in the electrons. c. evenly spread throughout the atom. b. ...
... Rutherford fired positively charged particles at metal foil and concluded that most of the mass of an atom was a. in the electrons. c. evenly spread throughout the atom. b. ...
The Role of Initial Conditions in the Decay of Spatially Periodic
... its factory doped version Phase 5A. This substance (a kind of a standard for EC measurements) is chemically stable and its material parameters on which all explicit calculations throughout this paper were based, are well known [10]. All measurements were carried out at the temperature T = 30.0 ± 0.0 ...
... its factory doped version Phase 5A. This substance (a kind of a standard for EC measurements) is chemically stable and its material parameters on which all explicit calculations throughout this paper were based, are well known [10]. All measurements were carried out at the temperature T = 30.0 ± 0.0 ...
Chemical-Principles-7th-Edition-Zumdahl-Test-Bank
... ANS: D PTS: 1 DIF: moderate TOP: 2.5 KEY: general chemistry | early atomic theory | atomic theory of matter | isotope 11. How many protons, neutrons, and electrons does the atom 31P have? A) 16 protons, 15 neutrons, 16 electrons B) 15 protons, 15 neutrons, 31 electrons C) 16 protons, 16 neutrons, 15 ...
... ANS: D PTS: 1 DIF: moderate TOP: 2.5 KEY: general chemistry | early atomic theory | atomic theory of matter | isotope 11. How many protons, neutrons, and electrons does the atom 31P have? A) 16 protons, 15 neutrons, 16 electrons B) 15 protons, 15 neutrons, 31 electrons C) 16 protons, 16 neutrons, 15 ...
Chm 1
... ____ 20. Atoms of the same element can differ in a. chemical properties. c. atomic number. b. mass number. d. number of protons and electrons. ____ 21. The average atomic mass of an element is the average of the atomic masses of its a. naturally occurring isotopes. c. radioactive isotopes. b. two mo ...
... ____ 20. Atoms of the same element can differ in a. chemical properties. c. atomic number. b. mass number. d. number of protons and electrons. ____ 21. The average atomic mass of an element is the average of the atomic masses of its a. naturally occurring isotopes. c. radioactive isotopes. b. two mo ...
Document
... The Must Be Something Else There! • to answer these questions, Rutherford proposed that there was another particle in the nucleus – it is called a neutron • neutrons have no charge and a mass of 1 amu the masses of the proton and neutron are both approximately 1 amu ...
... The Must Be Something Else There! • to answer these questions, Rutherford proposed that there was another particle in the nucleus – it is called a neutron • neutrons have no charge and a mass of 1 amu the masses of the proton and neutron are both approximately 1 amu ...
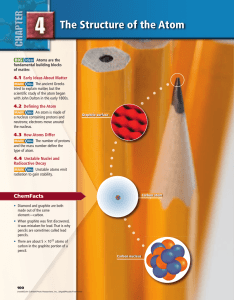
Chapter 4: The Structure of the Atom
... began to make connections between matter and electric charge. For instance, has your hair ever clung to your comb? To explore the connection, some scientists wondered how electricity might behave in the absence of matter. With the help of the newly invented vacuum pump, they passed electricity throu ...
... began to make connections between matter and electric charge. For instance, has your hair ever clung to your comb? To explore the connection, some scientists wondered how electricity might behave in the absence of matter. With the help of the newly invented vacuum pump, they passed electricity throu ...
1ST SEM MT CHAP 22 REVIEW
... How do radioactive nuclides affect photographic film wrapped in lightproof paper? a. They have no effect on the film. c. They melt the film. b. ...
... How do radioactive nuclides affect photographic film wrapped in lightproof paper? a. They have no effect on the film. c. They melt the film. b. ...
hty utI! rn h 1m 0 nt - Northside Middle School
... Was Dalton's atomic theory a huge step toward our current atomic model of matter? Yes. Was all of Dalton's theory accurate? No. As is often the case in science, Dalton's theory had to be revised as additional information was learned that could not be explained by the theory. As you will soon learn , ...
... Was Dalton's atomic theory a huge step toward our current atomic model of matter? Yes. Was all of Dalton's theory accurate? No. As is often the case in science, Dalton's theory had to be revised as additional information was learned that could not be explained by the theory. As you will soon learn , ...
Chapter 2: Atoms, Ions, and the Periodic Table
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
Chapter 2: Atoms, Ions, and the Periodic Table
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
Ch 1,2,4,25 pT
... Who was the man who lived from 460B.C.–370B.C. and was among the first to suggest the idea of atoms? a. Atomos c. Democritus b. Dalton d. Thomson The smallest particle of an element that retains the properties of that element is a(n) ____. a. atom c. proton b. electron d. neutron Which of the follow ...
... Who was the man who lived from 460B.C.–370B.C. and was among the first to suggest the idea of atoms? a. Atomos c. Democritus b. Dalton d. Thomson The smallest particle of an element that retains the properties of that element is a(n) ____. a. atom c. proton b. electron d. neutron Which of the follow ...
39 The Atomic Nucleus and Radioactivity
... Another factor that limits the stability of a nuclei is the instability of the neutron. A lone neutron will decay into a proton plus an electron (and also an antineutrino). About half of a bunch of lone neutrons will decay in 11 minutes. Particles that decay by spontaneously emitting charged particl ...
... Another factor that limits the stability of a nuclei is the instability of the neutron. A lone neutron will decay into a proton plus an electron (and also an antineutrino). About half of a bunch of lone neutrons will decay in 11 minutes. Particles that decay by spontaneously emitting charged particl ...
EPDG ILT Template - Nuclear Community
... neutron has merely bounced off, or scattered as it interacts with the nucleus. In some cases, the initial and final neutrons are not the same. There are two categories of scattering reactions, elastic, and inelastic scattering. These collisions are of great importance for the process of making therm ...
... neutron has merely bounced off, or scattered as it interacts with the nucleus. In some cases, the initial and final neutrons are not the same. There are two categories of scattering reactions, elastic, and inelastic scattering. These collisions are of great importance for the process of making therm ...
2. Nuclear magnetic resonance spectroscopy
... Because the electron distribution around chemically different hydrogen atoms in a molecule are not the same, the induced fields vary slightly. Conseqently, the nuclei experience different magnetic fields in the same external field. The effect is, however, very small; for hydrogen atoms it is only a ...
... Because the electron distribution around chemically different hydrogen atoms in a molecule are not the same, the induced fields vary slightly. Conseqently, the nuclei experience different magnetic fields in the same external field. The effect is, however, very small; for hydrogen atoms it is only a ...
Nuclear_Physics
... • We have previously discussed two fundamental forces of nature – gravitational and electromagnetic. • The electromagnetic force between a proton (+) and an electron (-) is 1039 greater than the gravitational forces between the two particles. • Therefore the electromagnetic forces are the only impor ...
... • We have previously discussed two fundamental forces of nature – gravitational and electromagnetic. • The electromagnetic force between a proton (+) and an electron (-) is 1039 greater than the gravitational forces between the two particles. • Therefore the electromagnetic forces are the only impor ...
Chemistry - Summative Practice and Review for Chapter 4 and 5
... 51. Write the electron configuration for chromium. ...
... 51. Write the electron configuration for chromium. ...
Chapter 2: Atoms, Ions, and the Periodic Table
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
Chapter 2: Atoms, Ions, and the Periodic Table
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
FREE Sample Here
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
FREE Sample Here
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
Chapter 2: Atoms, Ions, and the Periodic Table
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
Chapter 2: Atoms, Ions, and the Periodic Table
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
Chapter 10: Nuclear Physics
... fundamental forces of nature – gravitational and electromagnetic. • The electromagnetic force between a proton (+) and an electron (-) is 1039 greater than the gravitational forces between the two particles. • Therefore the electromagnetic forces are the only important forces on electrons and are re ...
... fundamental forces of nature – gravitational and electromagnetic. • The electromagnetic force between a proton (+) and an electron (-) is 1039 greater than the gravitational forces between the two particles. • Therefore the electromagnetic forces are the only important forces on electrons and are re ...