File - Ms. Francois` Chemistry Class
... (1) Positive charge is evenly spread throughout its volume (2) Negative charge is mainly concentrated in its nucleus (3) Mass is evenly spread throughout its volume (4) Volume is mainly empty space 2. In Rutherford’s gold foil experiments, some alpha particles were deflected from their original path ...
... (1) Positive charge is evenly spread throughout its volume (2) Negative charge is mainly concentrated in its nucleus (3) Mass is evenly spread throughout its volume (4) Volume is mainly empty space 2. In Rutherford’s gold foil experiments, some alpha particles were deflected from their original path ...
atomic mass
... nucleons together (E = m c2) The periodic table shows the average atomic masses, in amu. These masses are the weighted averages of the masses of all of the naturally occurring isotopes . © 2014 W. W. Norton Co., Inc. ...
... nucleons together (E = m c2) The periodic table shows the average atomic masses, in amu. These masses are the weighted averages of the masses of all of the naturally occurring isotopes . © 2014 W. W. Norton Co., Inc. ...
chapter 1–introduction to earth history
... Principle of Original Horizontality (4): Steno’s principle which states that most sedimentary particles settle from fluids under the influence of gravity. The sediment must then be deposited in layers that are nearly horizontal and parallel to the surface on which it is accumulating. Principle of Or ...
... Principle of Original Horizontality (4): Steno’s principle which states that most sedimentary particles settle from fluids under the influence of gravity. The sediment must then be deposited in layers that are nearly horizontal and parallel to the surface on which it is accumulating. Principle of Or ...
E = mc 2 - Gordon State College
... • are high-frequency electromagnetic radiation • are emitted when a nucleus in an excited state moves to a lower energy state • are more harmful than alpha or beta particles • are most penetrating because they have no mass or charge • are pure energy, greater per photon than in visible or ultraviole ...
... • are high-frequency electromagnetic radiation • are emitted when a nucleus in an excited state moves to a lower energy state • are more harmful than alpha or beta particles • are most penetrating because they have no mass or charge • are pure energy, greater per photon than in visible or ultraviole ...
Chapter 14
... electron. The proton stays in the nucleus, but the high energy electron is ejected (this is called beta radiation). During beta decay, the atomic number increases by one because one new proton is created. The mass number stays the same because the atom lost a neutron but gained a proton. Gamma decay ...
... electron. The proton stays in the nucleus, but the high energy electron is ejected (this is called beta radiation). During beta decay, the atomic number increases by one because one new proton is created. The mass number stays the same because the atom lost a neutron but gained a proton. Gamma decay ...
What are atoms?
... • In 1897, J. J. Thomson performed experiments that detected smaller particles within atoms. • In the early 1900s, Ernest Rutherford and James Chadwick revealed the nature of the dense center of an atom. • Today we have the electron cloud model. ...
... • In 1897, J. J. Thomson performed experiments that detected smaller particles within atoms. • In the early 1900s, Ernest Rutherford and James Chadwick revealed the nature of the dense center of an atom. • Today we have the electron cloud model. ...
radioactivity - the Scientia Review
... http://0.tqn.com/d/chemistry/1/0/8/d/1/Pe similar quantity of neutrons. riodicTableWallpaper.png ...
... http://0.tqn.com/d/chemistry/1/0/8/d/1/Pe similar quantity of neutrons. riodicTableWallpaper.png ...
CHAPTER 2 ATOMS, MOLECULES, AND IONS Questions
... neutrons, and protons are the key smaller parts of an atom. b. All atoms of hydrogen have 1 proton in the nucleus. Different isotopes of hydrogen have 0, 1, or 2 neutrons in the nucleus. Because we are talking about atoms, this implies a neutral charge, which dictates 1 electron present for all hydr ...
... neutrons, and protons are the key smaller parts of an atom. b. All atoms of hydrogen have 1 proton in the nucleus. Different isotopes of hydrogen have 0, 1, or 2 neutrons in the nucleus. Because we are talking about atoms, this implies a neutral charge, which dictates 1 electron present for all hydr ...
CHAPTER 2 ATOMS, MOLECULES, AND IONS Questions
... neutrons, and protons are the key smaller parts of an atom. b. All atoms of hydrogen have 1 proton in the nucleus. Different isotopes of hydrogen have 0, 1, or 2 neutrons in the nucleus. Because we are talking about atoms, this implies a neutral charge, which dictates 1 electron present for all hydr ...
... neutrons, and protons are the key smaller parts of an atom. b. All atoms of hydrogen have 1 proton in the nucleus. Different isotopes of hydrogen have 0, 1, or 2 neutrons in the nucleus. Because we are talking about atoms, this implies a neutral charge, which dictates 1 electron present for all hydr ...
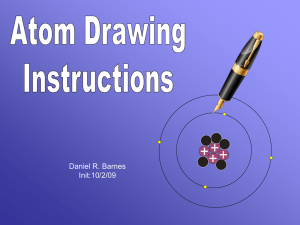
Atoms contain 3 particles
... Density and the Atom • Since most of the particles went through, it was mostly empty space. • Because the pieces turned so much, the positive pieces were heavy. • Small volume, big mass, big density. • This small dense positive area is the ...
... Density and the Atom • Since most of the particles went through, it was mostly empty space. • Because the pieces turned so much, the positive pieces were heavy. • Small volume, big mass, big density. • This small dense positive area is the ...
Atomic Mass Unit and Isotopes
... charge and fairly low penetrating power. Beta particles (β) – represented by 0-1β , are high speed electrons with a -1 charge and high penetrating power. Gamma rays (γ) – represented by 00γ , are ...
... charge and fairly low penetrating power. Beta particles (β) – represented by 0-1β , are high speed electrons with a -1 charge and high penetrating power. Gamma rays (γ) – represented by 00γ , are ...
Tamene Hailu - Addis Ababa University Institutional Repository
... Discovery of Neutron German physicist Bothe and Becker continuing Rutherford’s experiment on alpha particle bombardment of light atoms and observation of nuclear reaction discovered that when some elements such as ,lithium ,beryllium, or boron are exposed to alpha radiation there appears a strongly ...
... Discovery of Neutron German physicist Bothe and Becker continuing Rutherford’s experiment on alpha particle bombardment of light atoms and observation of nuclear reaction discovered that when some elements such as ,lithium ,beryllium, or boron are exposed to alpha radiation there appears a strongly ...
Chapter 14 REACTORS AND ACCELERATORS
... earth. The reasons that other nuclear reactions do not normally occur on earth are simple. Nuclear reactions that are induced by protons or heavier charged particles all have large activation barriers ...
... earth. The reasons that other nuclear reactions do not normally occur on earth are simple. Nuclear reactions that are induced by protons or heavier charged particles all have large activation barriers ...
Chapter 8
... • Chemical reactions occur when atoms are separated, joined, or rearranged. Atoms of one element never change into other kinds of atoms in chemical reactions. – Inaccuracies in Dalton’s Theory • Atoms are divisible. • Atoms of the same element aren’t entirely the same ...
... • Chemical reactions occur when atoms are separated, joined, or rearranged. Atoms of one element never change into other kinds of atoms in chemical reactions. – Inaccuracies in Dalton’s Theory • Atoms are divisible. • Atoms of the same element aren’t entirely the same ...
5 Early Atomic Theory and Structure Chapter Outline Early Theories
... Dalton’s theory of atoms, proposed in the early 1800s, states: 1. Elements are composed of small, indivisible particles called atoms. 2. Atoms of the same element are identical in mass and size. ...
... Dalton’s theory of atoms, proposed in the early 1800s, states: 1. Elements are composed of small, indivisible particles called atoms. 2. Atoms of the same element are identical in mass and size. ...
03_DetectOverview
... Recombination IC’s are prey to recombination, which results in an output current less than that given above. More important, recombination, if it is significant, depends on many factors and is therefore likely to vary with time. Current in IC’s is usually carried by positive ions going one way and ...
... Recombination IC’s are prey to recombination, which results in an output current less than that given above. More important, recombination, if it is significant, depends on many factors and is therefore likely to vary with time. Current in IC’s is usually carried by positive ions going one way and ...
Nuclear Reactions - Manasquan Public Schools
... isotopes of one element are changed, into stable isotopes of a different element. • Stable isotopes are not radioactive. ...
... isotopes of one element are changed, into stable isotopes of a different element. • Stable isotopes are not radioactive. ...
Structure of the Nuclear Atom
... Rutherford concluded that the atom is mostly empty space. All the positive charge and almost all of the mass are concentrated in a small region called the nucleus. ...
... Rutherford concluded that the atom is mostly empty space. All the positive charge and almost all of the mass are concentrated in a small region called the nucleus. ...
Atoms1 - Cbsephysicstutorials
... α-decay, there is a loss of 2 protons and 4 neutrons. In every β+-decay, there is a loss of 1 proton and a neutrino is emitted from the nucleus. In every β −-decay, there is a gain of 1 proton and an antineutrino is emitted from the nucleus. For the given cases, the various nuclear reactions can be ...
... α-decay, there is a loss of 2 protons and 4 neutrons. In every β+-decay, there is a loss of 1 proton and a neutrino is emitted from the nucleus. In every β −-decay, there is a gain of 1 proton and an antineutrino is emitted from the nucleus. For the given cases, the various nuclear reactions can be ...
II. Radioactive Decay
... • How was radioactivity discovered and studied? • What are the key properties of alpha, beta, and gamma radiation? ...
... • How was radioactivity discovered and studied? • What are the key properties of alpha, beta, and gamma radiation? ...
radioactive decay - Southwest High School
... • How was radioactivity discovered and studied? • What are the key properties of alpha, beta, and gamma radiation? ...
... • How was radioactivity discovered and studied? • What are the key properties of alpha, beta, and gamma radiation? ...
Chapter42015.1 STUDENT
... B. Elements are different kinds of atoms with a name, symbol, and unique properties. C. The Periodic Table lists the elements in the order based on the number of ___________________. D. The atomic number is written _________________the symbol and tells you the number of protons. E. The number of pro ...
... B. Elements are different kinds of atoms with a name, symbol, and unique properties. C. The Periodic Table lists the elements in the order based on the number of ___________________. D. The atomic number is written _________________the symbol and tells you the number of protons. E. The number of pro ...
isotopes
... We have talked in class about isotopes: atoms of the same element that have different numbers of neutrons in their nucleus. For example, there are actually three different kinds of hydrogen atoms. The most common atom (or isotope) of the element hydrogen is called protium. It has one proton and zero ...
... We have talked in class about isotopes: atoms of the same element that have different numbers of neutrons in their nucleus. For example, there are actually three different kinds of hydrogen atoms. The most common atom (or isotope) of the element hydrogen is called protium. It has one proton and zero ...
B - Cloudfront.net
... as much as they can, since they find each other’s positive electric charge to be quite repulsive. In fact, if it weren’t for the “strong force” holding the nucleons together, the protons would just fly out of the nucleus altogether, disgusted by each other’s positivity. ...
... as much as they can, since they find each other’s positive electric charge to be quite repulsive. In fact, if it weren’t for the “strong force” holding the nucleons together, the protons would just fly out of the nucleus altogether, disgusted by each other’s positivity. ...