Bucher Regents Review
... Metals: very dense, conductors, malleable, usually hard Non-metals: not very dense (often gases), poor conductors, soft or brittle solids 5. Elements can be differentiated by chemical properties. Chemical properties describe how an element behaves during a chemical reaction. Metals lose electrons (+ ...
... Metals: very dense, conductors, malleable, usually hard Non-metals: not very dense (often gases), poor conductors, soft or brittle solids 5. Elements can be differentiated by chemical properties. Chemical properties describe how an element behaves during a chemical reaction. Metals lose electrons (+ ...
CHAPTER 1, MATTER AND CHANGE Section 1, Chemistry Is a
... stable substances and is made of one type of atom. (Example: hydrogen) ! A compound is a substance that can be broken down into simple stable substances. Each compound is made from the atoms of two or more elements that are chemically bonded. (Example: hydrogen peroxide, H2O2) Properties and changes ...
... stable substances and is made of one type of atom. (Example: hydrogen) ! A compound is a substance that can be broken down into simple stable substances. Each compound is made from the atoms of two or more elements that are chemically bonded. (Example: hydrogen peroxide, H2O2) Properties and changes ...
DO NOW
... Read “The Bohr Model and Valence Electrons” from page 141. Take Cornell Notes, defining the following terms: - Bohr Model ...
... Read “The Bohr Model and Valence Electrons” from page 141. Take Cornell Notes, defining the following terms: - Bohr Model ...
Chapter Three: Atoms and Atomic Masses
... Probing Further into Atomic Structure: The Electron One of the three particles which make up all atoms is the electron Benjamin Franklin observed that, when a cloth was rubbed across a glass rod, a charge was developed on each. The charges, called positive (+) and negative (-), show an attract ...
... Probing Further into Atomic Structure: The Electron One of the three particles which make up all atoms is the electron Benjamin Franklin observed that, when a cloth was rubbed across a glass rod, a charge was developed on each. The charges, called positive (+) and negative (-), show an attract ...
Chemistry 127 – Chapter 2 Atoms, Molecules and Ions
... • An atom’s atomic mass can be determined to a highly precise value by using a high tech instrument known as a mass spectrometer. • The mass spectrometer separates matter based on its mass and charge. • This data can be used to determine the abundance and mass of each isotope in a naturally occurrin ...
... • An atom’s atomic mass can be determined to a highly precise value by using a high tech instrument known as a mass spectrometer. • The mass spectrometer separates matter based on its mass and charge. • This data can be used to determine the abundance and mass of each isotope in a naturally occurrin ...
Isotopes - Net Texts
... inside the atom. So if a neutron or two is added or removed from the nucleus, then the chemical properties will not change. This means that such an atom would remain in the same place in the periodic table. For example, no matter how many neutrons we add or subtract from a nucleus with 6 protons, th ...
... inside the atom. So if a neutron or two is added or removed from the nucleus, then the chemical properties will not change. This means that such an atom would remain in the same place in the periodic table. For example, no matter how many neutrons we add or subtract from a nucleus with 6 protons, th ...
2.1 Early Ideas in Atomic Theory
... of their day. In the fifth century BC, Leucippus and Democritus argued that all matter was composed of small, finite particles that they called atomos, a term derived from the Greek word for “indivisible.” They thought of atoms as moving particles that differed in shape and size, and which could joi ...
... of their day. In the fifth century BC, Leucippus and Democritus argued that all matter was composed of small, finite particles that they called atomos, a term derived from the Greek word for “indivisible.” They thought of atoms as moving particles that differed in shape and size, and which could joi ...
Lecture 02 Post. Rutherford Model
... • atomic masses of isotopes and elements • rudimentary knowledge of P.T. ...
... • atomic masses of isotopes and elements • rudimentary knowledge of P.T. ...
BS5-Ch 2.
... • Noble gases are especially stable • Main group elements will often gain or lose electrons to have the same number of electrons as the nearest noble gas • Metals form cations by losing electrons What is the expected charge on: Ca? 2+ Na? + ...
... • Noble gases are especially stable • Main group elements will often gain or lose electrons to have the same number of electrons as the nearest noble gas • Metals form cations by losing electrons What is the expected charge on: Ca? 2+ Na? + ...
AtomicStructure_Peri..
... Minerals: Calcium (chalk), Boron (borax)... The only thing you cannot name an element after is a living human. ...
... Minerals: Calcium (chalk), Boron (borax)... The only thing you cannot name an element after is a living human. ...
Unit 3 Atomics Review SCIENTIFIC THEORIES Dalton theorized that
... b. What the atomic mass of Carbon? ______________ c. If there are two isotopes of carbon, C-12 and C-14, which is more abundant? __________ d. Calculate the atomic mass of a sample of element X which contains 45% X-118 and the rest is X-120. ...
... b. What the atomic mass of Carbon? ______________ c. If there are two isotopes of carbon, C-12 and C-14, which is more abundant? __________ d. Calculate the atomic mass of a sample of element X which contains 45% X-118 and the rest is X-120. ...
Defining the Atom
... Rutherford concluded that the atom is mostly empty space. All the positive charge and almost all of the mass are concentrated in a small region called the nucleus. The nucleus is the tiny central core of an atom and is composed of protons and neutrons. ...
... Rutherford concluded that the atom is mostly empty space. All the positive charge and almost all of the mass are concentrated in a small region called the nucleus. The nucleus is the tiny central core of an atom and is composed of protons and neutrons. ...
File - Science by Shaw
... Protons and Neutrons All of an atom’s positive charge Almost all of an atom’s mass. ...
... Protons and Neutrons All of an atom’s positive charge Almost all of an atom’s mass. ...
Chapter 2
... in their attraction for valence electrons that one atom strips an electron completely from the other. • Example- sodium (one valence electron) in its third shell transfers this electron to chlorine with 7 valence electrons in its third shell. • Now, sodium has a full valence shell (the second) and c ...
... in their attraction for valence electrons that one atom strips an electron completely from the other. • Example- sodium (one valence electron) in its third shell transfers this electron to chlorine with 7 valence electrons in its third shell. • Now, sodium has a full valence shell (the second) and c ...
2nd Semester Chemistry Terms - Glancy 4TH PERIOD PHYSICAL
... 32. Nucleon- a nuclear proton or neutron 33. Half-life- the time required for half the atoms in a sample of a radioactive isotope to decay 34. Transmutation- the conversion of an atomic nucleus of one element into an atomic nucleus of another element through a loss or gain in the number of protons 3 ...
... 32. Nucleon- a nuclear proton or neutron 33. Half-life- the time required for half the atoms in a sample of a radioactive isotope to decay 34. Transmutation- the conversion of an atomic nucleus of one element into an atomic nucleus of another element through a loss or gain in the number of protons 3 ...
Atomic Structure
... Isotopes ▪ Isotopes are atoms that have the same number of protons but different numbers of neutrons. ▪They have different mass numbers, and may have different properties. ▪ Can write as either element or symbol – mass ▪Hydrogen-1 ▪H-2 ▪H-3 ...
... Isotopes ▪ Isotopes are atoms that have the same number of protons but different numbers of neutrons. ▪They have different mass numbers, and may have different properties. ▪ Can write as either element or symbol – mass ▪Hydrogen-1 ▪H-2 ▪H-3 ...
neutrons
... number due to varying numbers of neutrons Isotopes are usually identified by specifying their mass number. Two methods for specifying isotopes: The mass number is written with a hyphen after the name of the element ex: hydrogen-3 is tritium Show the composition of a nucleus as the isotopes nucle ...
... number due to varying numbers of neutrons Isotopes are usually identified by specifying their mass number. Two methods for specifying isotopes: The mass number is written with a hyphen after the name of the element ex: hydrogen-3 is tritium Show the composition of a nucleus as the isotopes nucle ...
Isotope Practice Worksheet
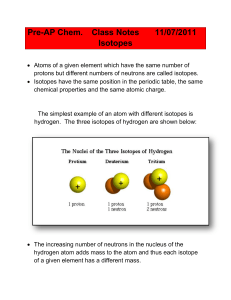
... Atoms of a given element which have the same number of protons but different numbers of neutrons are called isotopes. Isotopes have the same position in the periodic table, the same chemical properties and the same atomic charge. ...
... Atoms of a given element which have the same number of protons but different numbers of neutrons are called isotopes. Isotopes have the same position in the periodic table, the same chemical properties and the same atomic charge. ...
DEFINING THE ATOM - Southgate Schools
... ________ 11. The atomic number of an element is the sum of the protons and electrons in an atom of that element. ________ 12. The atomic number of an atom is the total number of protons in an atom of that element. ________ 13. An atom of nitrogen has 7 protons and 7 neutrons. ________ 14. Relative a ...
... ________ 11. The atomic number of an element is the sum of the protons and electrons in an atom of that element. ________ 12. The atomic number of an atom is the total number of protons in an atom of that element. ________ 13. An atom of nitrogen has 7 protons and 7 neutrons. ________ 14. Relative a ...
end of year review
... fluorine (F) _____13. Which of the following could be the molecular formula of a compound that has the ...
... fluorine (F) _____13. Which of the following could be the molecular formula of a compound that has the ...
Atom Notes Outline - Sewanhaka Central High School District
... 3. Find the following with regard to Nitrogen-14: Mass #: _______________ Atomic #: ______________ # of protons: ____________ # of neutrons: ____________ # of electrons: _____________ 4. An atom has 15 protons and 16 neutrons. What is its atomic #? _______________ What is its mass # ? ______________ ...
... 3. Find the following with regard to Nitrogen-14: Mass #: _______________ Atomic #: ______________ # of protons: ____________ # of neutrons: ____________ # of electrons: _____________ 4. An atom has 15 protons and 16 neutrons. What is its atomic #? _______________ What is its mass # ? ______________ ...