CHEM 120 WEEK 11 LECTURES (INORGANIC WEEK 2) Dr. MD
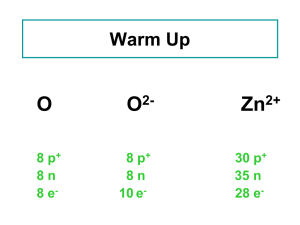
... Contains only metals, apart from boron. Boron is also the only element which does not form a stable trication (B3+) again will have too high a charge density to be stable. Why do the other elements form tri-cations (M3+ )? Soln. √ Because they have the valence electronic configuration ns2np1 and ...
... Contains only metals, apart from boron. Boron is also the only element which does not form a stable trication (B3+) again will have too high a charge density to be stable. Why do the other elements form tri-cations (M3+ )? Soln. √ Because they have the valence electronic configuration ns2np1 and ...
Isotopes-Chemistry
... Same Element Different AtomIsotopes All atoms of a particular element are not exactly alike. Some elements have atoms with different masses (isotopes) ...
... Same Element Different AtomIsotopes All atoms of a particular element are not exactly alike. Some elements have atoms with different masses (isotopes) ...
Bonds - Cloudfront.net
... What is lost is equally gained, therefore, the sum of the charges on the ions is zero ...
... What is lost is equally gained, therefore, the sum of the charges on the ions is zero ...
Ch#4 Atoms and Elements
... “intermingle” when atoms combine to form molecules. • It is the number of valence (furthest from nucleus) electrons that really determines chemical behavior. ...
... “intermingle” when atoms combine to form molecules. • It is the number of valence (furthest from nucleus) electrons that really determines chemical behavior. ...
Chem Midterm Review 2016
... Bohr Atom Niels Bohr quickly seized upon this used it to propose a quantized description of the atom. 1. Bohr proposed that while circling the nucleus of the atom, electrons could only occupy certain discrete orbits, that is to say energy levels. Bohr used Max Planck's equations describing quanta of ...
... Bohr Atom Niels Bohr quickly seized upon this used it to propose a quantized description of the atom. 1. Bohr proposed that while circling the nucleus of the atom, electrons could only occupy certain discrete orbits, that is to say energy levels. Bohr used Max Planck's equations describing quanta of ...
Unit 3 Chap. 3 Atoms: The Building Blocks of Matter
... Atom - the smallest particle of an element which possesses the properties of that element. Isotopes of an element are atoms with different numbers of neutrons in their nuclei. That is ,isotopes of an element have the same atomic numbers but different mass numbers. ...
... Atom - the smallest particle of an element which possesses the properties of that element. Isotopes of an element are atoms with different numbers of neutrons in their nuclei. That is ,isotopes of an element have the same atomic numbers but different mass numbers. ...
Ch. 2: Biochemistry
... Valence electrons: in the outermost shell, or valence shell Elements with full valence shell are chemically inert Chemical behavior of atom determined by distribution of electrons in electron shells, MOSTLY by valence electrons ...
... Valence electrons: in the outermost shell, or valence shell Elements with full valence shell are chemically inert Chemical behavior of atom determined by distribution of electrons in electron shells, MOSTLY by valence electrons ...
Inside an Atom
... electrons in the outer layer (furthest distance from nucleus) Valence electrons are very important because they are involved in the formation of chemical bonds A chemical bond forms between two atoms when valence electrons move between them. The valence electrons may be ...
... electrons in the outer layer (furthest distance from nucleus) Valence electrons are very important because they are involved in the formation of chemical bonds A chemical bond forms between two atoms when valence electrons move between them. The valence electrons may be ...
3. Atomic Structure and the Periodic Table
... clearly for the first time the existence of atoms. This was necessary to explain the fixed properties of an element. Third postulate was necessary to explain the existence of compounds and the breaking of compounds into elements. Fourth postulate was necessary to define and describe the chemical rea ...
... clearly for the first time the existence of atoms. This was necessary to explain the fixed properties of an element. Third postulate was necessary to explain the existence of compounds and the breaking of compounds into elements. Fourth postulate was necessary to define and describe the chemical rea ...
chem 1 TIFF new.indd
... As we just saw, the atomic number tells how many protons the atom contains. In an atom, the number of protons equals the number of electrons, so this number is also the number of electrons in an atom. For example, the smallest element is hydrogen. It has an atomic number of 1, which means it has onl ...
... As we just saw, the atomic number tells how many protons the atom contains. In an atom, the number of protons equals the number of electrons, so this number is also the number of electrons in an atom. For example, the smallest element is hydrogen. It has an atomic number of 1, which means it has onl ...
Document
... How do isotopes of the same element differ? How are they the same? What are the two ways to write isotopes? Write both ways for boron (B) atomic number 5 and mass 11 ...
... How do isotopes of the same element differ? How are they the same? What are the two ways to write isotopes? Write both ways for boron (B) atomic number 5 and mass 11 ...
Atomic Structure
... Table is determined by its proton number. All elements in the same group have the same number of valence electrons, which is the same as the Group number. All elements in the same period have the same number of electron shells. ...
... Table is determined by its proton number. All elements in the same group have the same number of valence electrons, which is the same as the Group number. All elements in the same period have the same number of electron shells. ...
Chapter 4
... Science as we know it did not exist several thousand years ago. Curiosity sparked the investigations of most scholarly thinkers and they based their thoughts on life experiences. The prefix “phil” means loving or dear & is seen in many science & nonscience words. “Philosophy” refers to a love of k ...
... Science as we know it did not exist several thousand years ago. Curiosity sparked the investigations of most scholarly thinkers and they based their thoughts on life experiences. The prefix “phil” means loving or dear & is seen in many science & nonscience words. “Philosophy” refers to a love of k ...
Topic 3: Periodicity
... two 4s electrons, which means that they all have an oxidation state of +2 ...
... two 4s electrons, which means that they all have an oxidation state of +2 ...
The periodic table
... • Valence shell = The outermost shell of e- . • The first e- orbital only holds 2. • All of the others we will consider hold UP TO 8. • (There are energy levels that hold more than 8 e- , but you’ll get to that in chemistry). ...
... • Valence shell = The outermost shell of e- . • The first e- orbital only holds 2. • All of the others we will consider hold UP TO 8. • (There are energy levels that hold more than 8 e- , but you’ll get to that in chemistry). ...
Chapter 2. The Chemical Context of Life
... Pair of electrons not shared equally by 2 atoms Water = O + H oxygen has stronger “attraction” for the shared electrons than hydrogen oxygen has higher ...
... Pair of electrons not shared equally by 2 atoms Water = O + H oxygen has stronger “attraction” for the shared electrons than hydrogen oxygen has higher ...
Matter – Properties and Changes
... The atomic mass of an element is the weighted average mass of the isotopes of that element. Chemists have taken samples of the different isotopes of a given element, for example Potassium-39, Potassium40, and Potassium-41, and have averaged the masses to determine atomic mass. That is different from ...
... The atomic mass of an element is the weighted average mass of the isotopes of that element. Chemists have taken samples of the different isotopes of a given element, for example Potassium-39, Potassium40, and Potassium-41, and have averaged the masses to determine atomic mass. That is different from ...
Ch#4 Atoms and Elements
... • Compound – distinct substance that is composed of the atoms of two or more elements and always contains exactly the same whole number ratio of those elements. • Chemical Formulas – expresses the types of atoms and the number of each type in each formula unit, or molecule of a given compound. ...
... • Compound – distinct substance that is composed of the atoms of two or more elements and always contains exactly the same whole number ratio of those elements. • Chemical Formulas – expresses the types of atoms and the number of each type in each formula unit, or molecule of a given compound. ...
Scientific Method - Virtual Medical Academy
... * fractionating column - part of apparatus where separation occurs. ...
... * fractionating column - part of apparatus where separation occurs. ...
Lesson 1 - Working With Chemicals
... o A nucleus – a central region that is positively charged and contains most of the mass - protons are heavy positive particles within the nucleus o Electrons – particles with a negative charge and are very light (compared to protons). - Electrons circle around the nucleus o Empty space surrounding t ...
... o A nucleus – a central region that is positively charged and contains most of the mass - protons are heavy positive particles within the nucleus o Electrons – particles with a negative charge and are very light (compared to protons). - Electrons circle around the nucleus o Empty space surrounding t ...
Scientific Method - Virtual Medical Academy
... * fractionating column - part of apparatus where separation occurs. ...
... * fractionating column - part of apparatus where separation occurs. ...
13.2 Chemical Formulas
... What is a chemical formula? Chemical formulas have two important parts: chemical symbols for the elements in the compound and subscripts that tell how many atoms of each element are needed to form the compound. The chemical formula for water, H2O, tells us that a water molecule is made of the elemen ...
... What is a chemical formula? Chemical formulas have two important parts: chemical symbols for the elements in the compound and subscripts that tell how many atoms of each element are needed to form the compound. The chemical formula for water, H2O, tells us that a water molecule is made of the elemen ...
The Atom Powerpoint 10-16-13
... The ATOMIC MASS NUMBER reported for an element on the PERIODIC TABLE is an AVERAGE of all the different ISOTOPES of that element. ...
... The ATOMIC MASS NUMBER reported for an element on the PERIODIC TABLE is an AVERAGE of all the different ISOTOPES of that element. ...
end of year review
... _____ 4. Which of the following correctly pairs a phase of matter with its description? A. Solid: Particles have no motion. B. Liquid: Particles expand to fill any container in which they are placed. C. ...
... _____ 4. Which of the following correctly pairs a phase of matter with its description? A. Solid: Particles have no motion. B. Liquid: Particles expand to fill any container in which they are placed. C. ...