Chapter 2 Atoms and Ions
... let him see if any alpha-particles can be scattered through a large angle?" I may tell you in confidence that I did not believe that they would be, since we knew the alpha-particle was a very fast, massive particle with a great deal of energy, and you could show that if the scattering was due to the ...
... let him see if any alpha-particles can be scattered through a large angle?" I may tell you in confidence that I did not believe that they would be, since we knew the alpha-particle was a very fast, massive particle with a great deal of energy, and you could show that if the scattering was due to the ...
The Atom
... Greeks settled disagreements by argument Aristotle was more famous He won His ideas carried through middle ages. Alchemists change lead to gold ...
... Greeks settled disagreements by argument Aristotle was more famous He won His ideas carried through middle ages. Alchemists change lead to gold ...
KEY Midterm Exam 1 Sept.14, 1999 Chemistry 211 PAGE 1 0f 5
... (a) atoms with different numbers of protons and neutrons. (b) atoms with the same number of neutrons and electrons. (c) atoms with the same atomic number and different mass numbers. (d) atoms with the same mass number but different atomic numbers. ...
... (a) atoms with different numbers of protons and neutrons. (b) atoms with the same number of neutrons and electrons. (c) atoms with the same atomic number and different mass numbers. (d) atoms with the same mass number but different atomic numbers. ...
Atomic - Ms. Dawkins
... What is the structure of an atom? • The protons and neutrons are grouped together in the center of the atom. • The center of the atom is called the nucleus. • Electrons move around outside the nucleus in what we call an electron cloud. • The nucleus has an overall positive charge (because it contai ...
... What is the structure of an atom? • The protons and neutrons are grouped together in the center of the atom. • The center of the atom is called the nucleus. • Electrons move around outside the nucleus in what we call an electron cloud. • The nucleus has an overall positive charge (because it contai ...
Chapter 4 Atoms and Elements
... the nuclear theory of the atom. 1. Most of the atom’s mass and all of its positive charge are contained in a small core called the nucleus. 2. Most of the volume of the atom is empty space through which the tiny, negatively charged electrons are dispersed. 3. The number of negatively charged electro ...
... the nuclear theory of the atom. 1. Most of the atom’s mass and all of its positive charge are contained in a small core called the nucleus. 2. Most of the volume of the atom is empty space through which the tiny, negatively charged electrons are dispersed. 3. The number of negatively charged electro ...
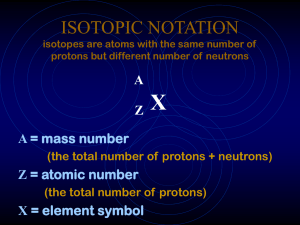
ISOTOPIC NOTATION isotopes are atoms with the same number
... • Generally the formula used is: % X + % Y + % Z… = atomic mass. An instrument called the mass spectrometer is generally used to determine the percentages and individual masses of each isotope. ...
... • Generally the formula used is: % X + % Y + % Z… = atomic mass. An instrument called the mass spectrometer is generally used to determine the percentages and individual masses of each isotope. ...
3-3 Molar Mass
... Atomic mass unit – exactly 1/12 the mass of a carbon-12 atom Atomic mass of any other atom is determined by comparing it with the mass of carbon-12 ...
... Atomic mass unit – exactly 1/12 the mass of a carbon-12 atom Atomic mass of any other atom is determined by comparing it with the mass of carbon-12 ...
atomic mass
... C-12 is used as the reference for atomic masses. One atom of C-12 has a mass of exactly 12 amu ...
... C-12 is used as the reference for atomic masses. One atom of C-12 has a mass of exactly 12 amu ...
Chapter 3
... subatomic particles are very small when measured in grams. Atomic masses are expressed on a relative mass scale. One atom is assigned a mass, and all others are measured relative to it. ▶ The basis for the relative atomic mass scale is an atom of carbon that contains 6 protons and 6 neutrons. This c ...
... subatomic particles are very small when measured in grams. Atomic masses are expressed on a relative mass scale. One atom is assigned a mass, and all others are measured relative to it. ▶ The basis for the relative atomic mass scale is an atom of carbon that contains 6 protons and 6 neutrons. This c ...
Explain APE MAN NOTES TEACHER PAGE
... Ex.) Fluorine has an atomic number of 9. That means there are nine positively charged protons. Since it is a neutral atom, it also has 8 negatively charged electrons. ...
... Ex.) Fluorine has an atomic number of 9. That means there are nine positively charged protons. Since it is a neutral atom, it also has 8 negatively charged electrons. ...
Chemistry in Biology
... A. Elements—pure substances that cannot be broken down into simpler kinds of matter • Made of one type of atom • More than 100 elements (92 naturally occurring) • 90% of the mass of an organism is composed of 4 elements (oxygen, carbon, hydrogen, and nitrogen) • Each element has a unique chemical sy ...
... A. Elements—pure substances that cannot be broken down into simpler kinds of matter • Made of one type of atom • More than 100 elements (92 naturally occurring) • 90% of the mass of an organism is composed of 4 elements (oxygen, carbon, hydrogen, and nitrogen) • Each element has a unique chemical sy ...
chemical bonds - geraldinescience
... Chemical Formulas • A chemical formula is a combination of letters and numbers that shows which elements make up a compound and the number of atoms of each element that are required to make a molecule of a compound. • In a chemical formula, the subscript that appears after the symbol for an element ...
... Chemical Formulas • A chemical formula is a combination of letters and numbers that shows which elements make up a compound and the number of atoms of each element that are required to make a molecule of a compound. • In a chemical formula, the subscript that appears after the symbol for an element ...
chap 3 notes
... charged plates above and below the tube. Found that the beam of radiation bends toward the positively charged plate, and away from the ...
... charged plates above and below the tube. Found that the beam of radiation bends toward the positively charged plate, and away from the ...
In an atom
... second shell about four kilometers, the third nine kilometers and so on. If you find that hard to visualize then try this. The period at the end of this sentence, (depending on your monitor and the font you are using), is probably about 1/2 a millimeter in diameter. If that period represents the nuc ...
... second shell about four kilometers, the third nine kilometers and so on. If you find that hard to visualize then try this. The period at the end of this sentence, (depending on your monitor and the font you are using), is probably about 1/2 a millimeter in diameter. If that period represents the nuc ...
Name Honors Chemistry ___/___/___ Subatomic Particles Atomic
... The atomic weight of an element is the weighted average of the masses of the isotopes of that element. The weighted average is determined using the abundance and mass of each isotope. Most elements have more than one naturally occurring isotope. For example, there are two naturally occurring isotope ...
... The atomic weight of an element is the weighted average of the masses of the isotopes of that element. The weighted average is determined using the abundance and mass of each isotope. Most elements have more than one naturally occurring isotope. For example, there are two naturally occurring isotope ...
The atom CP and H ONLINE
... If something has 5 protons, 5 neutrons, and 5 electrons, what is the mass number? 5 P + 5 N= 10 amu total Remember, mass number= P + N ...
... If something has 5 protons, 5 neutrons, and 5 electrons, what is the mass number? 5 P + 5 N= 10 amu total Remember, mass number= P + N ...
module for international standard class
... F. Growth of Atomic Theory a. Dalton Theory - All matter is made up of small particles called atoms - All atoms of a given type are similar to each other and significantly different from all other types - The relative number and arrangement of the different types of atoms found within o pure substa ...
... F. Growth of Atomic Theory a. Dalton Theory - All matter is made up of small particles called atoms - All atoms of a given type are similar to each other and significantly different from all other types - The relative number and arrangement of the different types of atoms found within o pure substa ...
Atomic Theory WebQuest PDF
... All atoms of the same element are exactly the same. Atoms of different elements are different. ...
... All atoms of the same element are exactly the same. Atoms of different elements are different. ...
Balancing Chemical Equations
... • The reactant chemical(s) are given on the left-hand side and the product chemical(s) on the right-hand side. ...
... • The reactant chemical(s) are given on the left-hand side and the product chemical(s) on the right-hand side. ...
Atomic Theory Webquest
... All atoms of the same element are exactly the same. Atoms of different elements are different. ...
... All atoms of the same element are exactly the same. Atoms of different elements are different. ...
File - Mrs. Hille`s FunZone
... Atoms of the same element are identical, those of different atoms are different. Atoms of different elements combine in whole number ratios to form compounds Chemical reactions involve the rearrangement of atoms. No new atoms are created or destroyed. ...
... Atoms of the same element are identical, those of different atoms are different. Atoms of different elements combine in whole number ratios to form compounds Chemical reactions involve the rearrangement of atoms. No new atoms are created or destroyed. ...
Name: Date: Chemistry 1 – Midterm Review Sheet Unit 1 – Scientific
... e. rusting iron 4. How many of the following are compounds: table salt, carbon, copper, water, mercury? a. 1 b. 2 c. 3 d. 4 e. 5 ...
... e. rusting iron 4. How many of the following are compounds: table salt, carbon, copper, water, mercury? a. 1 b. 2 c. 3 d. 4 e. 5 ...
Chemistry - Halifax County Public Schools
... add distilled water directly to the acid add acid to the distilled water first heat the acid and then add acid to the distilled water first heat the acid and then add distilled water to the acid ...
... add distilled water directly to the acid add acid to the distilled water first heat the acid and then add acid to the distilled water first heat the acid and then add distilled water to the acid ...
Key Concept 1: An atom is the smallest unit of an element that
... Key Concept 10: The reactivity of an atom is how easily and readily its valence electrons interact with the valence electrons of other atoms. Atoms of metals have a tendency to transfer electrons to nonmetals when they react. Atoms of nonmetals have a tendency to gain or share electrons when they re ...
... Key Concept 10: The reactivity of an atom is how easily and readily its valence electrons interact with the valence electrons of other atoms. Atoms of metals have a tendency to transfer electrons to nonmetals when they react. Atoms of nonmetals have a tendency to gain or share electrons when they re ...
key concepts of matter
... Key Concept 1: During a chemical reaction, the atoms of substances rearrange themselves into a new configuration forming new substances. The reactants (or the energy and atoms or molecules of the original substance) combine to produce products (or the energy, atoms, and molecules of the new substanc ...
... Key Concept 1: During a chemical reaction, the atoms of substances rearrange themselves into a new configuration forming new substances. The reactants (or the energy and atoms or molecules of the original substance) combine to produce products (or the energy, atoms, and molecules of the new substanc ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.