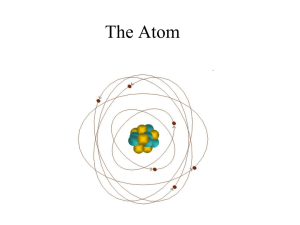
Atomic Theory- 1. Matter is composed of tiny, indivisible particles
... Atomic Theory1. Matter is composed of tiny, indivisible particles called atoms. 2. An element is composed of one type of atom. Properties of atoms are identical to each other. 3. A compound contains two or more different elements. The relative number of atoms of each element in a compound is the sam ...
... Atomic Theory1. Matter is composed of tiny, indivisible particles called atoms. 2. An element is composed of one type of atom. Properties of atoms are identical to each other. 3. A compound contains two or more different elements. The relative number of atoms of each element in a compound is the sam ...
Chemistry 1 – Tollett Chapter 5 – Atomic Structure & The Periodic
... been discovered, Dalton’s model of the atom had to be modified. ...
... been discovered, Dalton’s model of the atom had to be modified. ...
levels of organization and the atom
... The subatomic particles that make up the atoms are protons, neutrons, and electrons. Strong forces bind protons and neutrons together to form the nucleus, which is at the center of the atom. Here is the atom’s mass. Protons and neutrons have the same mass, 1 atomic mass unit (amu). However, protons ...
... The subatomic particles that make up the atoms are protons, neutrons, and electrons. Strong forces bind protons and neutrons together to form the nucleus, which is at the center of the atom. Here is the atom’s mass. Protons and neutrons have the same mass, 1 atomic mass unit (amu). However, protons ...
Ch 1.1 ppt
... • Certain chemical properties were repeated regularly. • These properties were related to the sum of the protons and neutrons in an atom. ...
... • Certain chemical properties were repeated regularly. • These properties were related to the sum of the protons and neutrons in an atom. ...
element - Mrs. Phillips` Physical Science Webpage
... • When you add energy to an atom, that energy can excite the electrons, causing them to jump out to a farther energy level. This is not a stable arrangement, and another electron will likely ‘fall’ down into the vacated spot in the lower orbital. When it drops down, it loses energy that it no longer ...
... • When you add energy to an atom, that energy can excite the electrons, causing them to jump out to a farther energy level. This is not a stable arrangement, and another electron will likely ‘fall’ down into the vacated spot in the lower orbital. When it drops down, it loses energy that it no longer ...
The Atom - Riverside City College
... Lithium has two natural isotopes, 6Li and 7Li, which have percent abundances of 7.5% and 92.5% respectively. What is the average atomic mass of Lithium? 2) Using the percent abundances below, calculate the average atomic mass for ...
... Lithium has two natural isotopes, 6Li and 7Li, which have percent abundances of 7.5% and 92.5% respectively. What is the average atomic mass of Lithium? 2) Using the percent abundances below, calculate the average atomic mass for ...
Chemistry--Chapter 5: Atomic Structure and the Periodic Table
... one elements’ atoms from another; small whole numbers on periodic table B. Mass Number--the total number of protons and neutrons (not found on p.t.), the “14” in carbon-14 C. Isotopes--elements of the same atom that have a different number of neutrons, so a different mass number D. Atomic Mass 1. Nu ...
... one elements’ atoms from another; small whole numbers on periodic table B. Mass Number--the total number of protons and neutrons (not found on p.t.), the “14” in carbon-14 C. Isotopes--elements of the same atom that have a different number of neutrons, so a different mass number D. Atomic Mass 1. Nu ...
World of
... Rules for Writing Formulas 1.Each atom present is represented by its element symbol. 2.The number of each type of atom is indicated by a subscript written to the right of the element symbol. 3.When only one atom of a given type is present, the subscript 1 is not written. Copyright© by Houghton Miff ...
... Rules for Writing Formulas 1.Each atom present is represented by its element symbol. 2.The number of each type of atom is indicated by a subscript written to the right of the element symbol. 3.When only one atom of a given type is present, the subscript 1 is not written. Copyright© by Houghton Miff ...
Chemistry Study Guide
... The first version of the modern periodic table was created by Dmitri Mendeleev. He was Russian chemist that classified matter based on physical and chemical properties. He organized the known elements of the time by increasing atomic mass. He left gaps in his table where he believed new elements tha ...
... The first version of the modern periodic table was created by Dmitri Mendeleev. He was Russian chemist that classified matter based on physical and chemical properties. He organized the known elements of the time by increasing atomic mass. He left gaps in his table where he believed new elements tha ...
Reading Comprehension - Easy Peasy All-in
... Everything around you is made of matter. Matter is made of at least one element. An element is made of atoms that are all the same kind. It is a pure form of matter. Elements join together with other elements to make the different materials that we see and use every day. Some common elements that yo ...
... Everything around you is made of matter. Matter is made of at least one element. An element is made of atoms that are all the same kind. It is a pure form of matter. Elements join together with other elements to make the different materials that we see and use every day. Some common elements that yo ...
Atomic terms - ATOMIC NUMBER: The number of protons in the
... - poor conductors of heat and electricity. Most nonmetals do not conduct well at all (insulators) - many of the nonmetals are gases at room temperature. A few solids, and one liquid (bromine) - color: Nonmetals may be white, black, purple, green, blue, orange, or colorless etc. - usually have low me ...
... - poor conductors of heat and electricity. Most nonmetals do not conduct well at all (insulators) - many of the nonmetals are gases at room temperature. A few solids, and one liquid (bromine) - color: Nonmetals may be white, black, purple, green, blue, orange, or colorless etc. - usually have low me ...
TERM 2 Unit 3 YR 9 SCI It is elementary
... according to currently accepted understandings. They will identify patterns in atomic structure that allow prediction of the products of chemical reactions and are reflected by the periodic table. They recognise that new substances are formed during a chemical reaction, and are able to list and desc ...
... according to currently accepted understandings. They will identify patterns in atomic structure that allow prediction of the products of chemical reactions and are reflected by the periodic table. They recognise that new substances are formed during a chemical reaction, and are able to list and desc ...
The parts of Dalton`s theory Matter is composed of small, chemically
... ELEMENTS are kinds of matter that contain only a single kind of atom. All the atoms of an element have identical chemical properties. COMPOUNDS are kinds of matter that are composed of atoms of two or more ELEMENTS which are combined in simple, whole number ratios. Most importantly, CHEMICAL REACTIO ...
... ELEMENTS are kinds of matter that contain only a single kind of atom. All the atoms of an element have identical chemical properties. COMPOUNDS are kinds of matter that are composed of atoms of two or more ELEMENTS which are combined in simple, whole number ratios. Most importantly, CHEMICAL REACTIO ...
Radioisotopes
... • Isotopes are any of the different types of atoms (Nuclides) of the same chemical element, each having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have di ...
... • Isotopes are any of the different types of atoms (Nuclides) of the same chemical element, each having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have di ...
Chapter 3
... Developed a new diagram of the atom Electrons can only be at certain energies Electrons must gain a specific amount of energy to move to a higher level, called a quantum ...
... Developed a new diagram of the atom Electrons can only be at certain energies Electrons must gain a specific amount of energy to move to a higher level, called a quantum ...
Intro to Element Note Answers
... Whose idea is closer to what we believe today? Democritus Whose idea was widely accepted for ~2000 years? Aristotle ...
... Whose idea is closer to what we believe today? Democritus Whose idea was widely accepted for ~2000 years? Aristotle ...
Name: Chapter 4 and 5 Study Guide Who was the Greek
... 16. What is going on inside the atoms when a neon light glows? 17. In a periodic table, a set of properties repeats from… a. Element to element b. Group to group c. Column to column d. Row to row 18. The usefulness of Mendeleev’s periodic table was confirmed by… a. The discovery of subatomic particl ...
... 16. What is going on inside the atoms when a neon light glows? 17. In a periodic table, a set of properties repeats from… a. Element to element b. Group to group c. Column to column d. Row to row 18. The usefulness of Mendeleev’s periodic table was confirmed by… a. The discovery of subatomic particl ...
Chapter 5
... o Using carbon-12 as the standard, an atomic mass unit (amu) is defined as one twelfth of the mass of a carbon-12 atom o In nature, most elements occur as a mixture of two or more isotopes o Atomic mass is the average mass of the atoms in a naturally occurring sample of the element - To calculate th ...
... o Using carbon-12 as the standard, an atomic mass unit (amu) is defined as one twelfth of the mass of a carbon-12 atom o In nature, most elements occur as a mixture of two or more isotopes o Atomic mass is the average mass of the atoms in a naturally occurring sample of the element - To calculate th ...
Basic Chemistry Lesson - Agriculture Solutions
... During the course you will often hear us refer to elements. An element is a substance made up of a single type of atom. To distinguish between elements, each element is assigned an atomic number (we rarely refer to these numbers during the course). The atomic number is the number of protons in the n ...
... During the course you will often hear us refer to elements. An element is a substance made up of a single type of atom. To distinguish between elements, each element is assigned an atomic number (we rarely refer to these numbers during the course). The atomic number is the number of protons in the n ...
(null): 096.AtomReview
... a. Each specific color tells us about the structure of specific electrons inside the atom b. Zinc spectrum s different from ANY other element (compare to sulfur and helium on same slide) E. Atomic structure basics (see AtomOverview.ppt) – what we’ve learned by seeing without seeing … 1. PROTONS: a. ...
... a. Each specific color tells us about the structure of specific electrons inside the atom b. Zinc spectrum s different from ANY other element (compare to sulfur and helium on same slide) E. Atomic structure basics (see AtomOverview.ppt) – what we’ve learned by seeing without seeing … 1. PROTONS: a. ...
Chapter 5
... – Tells number of protons and electrons Mass Number -On the periodic table -unit = AMU (atomic mass units) -Number of protons + neutrons -to find the number of neutrons, MASS NUMBER – ATOMIC NUMBER ...
... – Tells number of protons and electrons Mass Number -On the periodic table -unit = AMU (atomic mass units) -Number of protons + neutrons -to find the number of neutrons, MASS NUMBER – ATOMIC NUMBER ...
File
... the first compound to its mass in the second compound, (as it combines with the same mass of the other element), can always be expressed as ratios of small whole numbers( ex: 1:3 or 2:5). ...
... the first compound to its mass in the second compound, (as it combines with the same mass of the other element), can always be expressed as ratios of small whole numbers( ex: 1:3 or 2:5). ...
Chapter 4 Structure of the Atom An atom is the smallest particle of an
... Democritus is the first person to define an atom. Greek philosopher 2000 years ago. Atom is so small and indestructible that it cannot be divided up into anything smaller. Atom means indivisible in Greek. Law of conservation of mass – ...
... Democritus is the first person to define an atom. Greek philosopher 2000 years ago. Atom is so small and indestructible that it cannot be divided up into anything smaller. Atom means indivisible in Greek. Law of conservation of mass – ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.