Atoms, Elements, and the Periodic Table Part 1: The Atomic Model
... Rutherford discovered protons and the nucleus. He showed that atoms have (+) particles in the center, and are mostly empty space. ...
... Rutherford discovered protons and the nucleus. He showed that atoms have (+) particles in the center, and are mostly empty space. ...
Atoms, Elements, and the Periodic Table Part 1: The Atomic Model
... Rutherford discovered protons and the nucleus. He showed that atoms have (+) particles in the center, and are mostly empty space. ...
... Rutherford discovered protons and the nucleus. He showed that atoms have (+) particles in the center, and are mostly empty space. ...
Elements, Compounds, Mixtures
... groups of atoms bonded together, called molecules. • All matter can be classified as either a pure substance or a mixture. • A chemical substance is a form of matter that has a definite chemical composition and distinct ...
... groups of atoms bonded together, called molecules. • All matter can be classified as either a pure substance or a mixture. • A chemical substance is a form of matter that has a definite chemical composition and distinct ...
Elements and Atoms
... 3. Electrons (e-) = (negative charge) move nearly the speed of light form a cloud around the nucleus ...
... 3. Electrons (e-) = (negative charge) move nearly the speed of light form a cloud around the nucleus ...
Lecture 2: Atoms - U of L Class Index
... Mass number (A) = # protons + # neutrons Atomic number (Z) = # protons ...
... Mass number (A) = # protons + # neutrons Atomic number (Z) = # protons ...
SCH3U Course Review
... 10. Explain and illustrate the variation of the following in the Periodic Table: (a) ionization energy (b) electron affinity (c) atomic radius 11. For the following elements: (a) state if they tend to gain or lose electrons (b) how many electrons are gained or lost. (a) H (b) Na (c) Cl (d) Mg (e) S ...
... 10. Explain and illustrate the variation of the following in the Periodic Table: (a) ionization energy (b) electron affinity (c) atomic radius 11. For the following elements: (a) state if they tend to gain or lose electrons (b) how many electrons are gained or lost. (a) H (b) Na (c) Cl (d) Mg (e) S ...
and the atomic
... around the nucleus • based on information about how the energy of an atom changes when it absorbs and ...
... around the nucleus • based on information about how the energy of an atom changes when it absorbs and ...
Average Atomic Mass
... Average Atomic Mass - the weighted average of the masses of the isotopes of the element. Average Atomic Mass = [(isotope mass)(percent abundance)]/100% To solve for percent abundance assign the first isotope percentage x and the second isotope percentage equal to 100% - x 49. There are two natura ...
... Average Atomic Mass - the weighted average of the masses of the isotopes of the element. Average Atomic Mass = [(isotope mass)(percent abundance)]/100% To solve for percent abundance assign the first isotope percentage x and the second isotope percentage equal to 100% - x 49. There are two natura ...
The parts of Dalton`s theory Matter is composed of small, chemically
... ELEMENTS are kinds of matter that contain only a single kind of atom. All the atoms of an element have identical chemical properties. COMPOUNDS are kinds of matter that are composed of atoms of two or more ELEMENTS which are combined in simple, whole number ratios. Most importantly, CHEMICAL REACTIO ...
... ELEMENTS are kinds of matter that contain only a single kind of atom. All the atoms of an element have identical chemical properties. COMPOUNDS are kinds of matter that are composed of atoms of two or more ELEMENTS which are combined in simple, whole number ratios. Most importantly, CHEMICAL REACTIO ...
The_Atoms_Family
... • A substance that cannot be broken down into simpler substances by physical or chemical means • 92 occur naturally on the Earth and in stars • Each is identified by a one- , two-, or three-letter abbreviation • Some elements known in ancient times like gold and mercury, have symbols that reflect th ...
... • A substance that cannot be broken down into simpler substances by physical or chemical means • 92 occur naturally on the Earth and in stars • Each is identified by a one- , two-, or three-letter abbreviation • Some elements known in ancient times like gold and mercury, have symbols that reflect th ...
MORE ABOUT PROTONS, NEUTRONS AND ELECTRONS
... carbon for instance, have a mass of 12 a.m.u. and some have a mass of 14 a.m.u. All carbon atoms have the same number of protons and electrons but the numbers of neutrons differ. Atoms of the same element but with different atomic masses are called ISOTOPES. Most elements have some naturally occurin ...
... carbon for instance, have a mass of 12 a.m.u. and some have a mass of 14 a.m.u. All carbon atoms have the same number of protons and electrons but the numbers of neutrons differ. Atoms of the same element but with different atomic masses are called ISOTOPES. Most elements have some naturally occurin ...
Atomic Structure AKS Correlation Use the modern atomic theory to
... Atoms mostly about matter Matter cannot be empty space, dense and its’ makecreated or center up of atoms destroyed Atoms of same element look the same Chem reactions occur when atoms are rearranged Compounds formed by 2 or more atoms Describe the basic structure of the atom as protons, neutrons and ...
... Atoms mostly about matter Matter cannot be empty space, dense and its’ makecreated or center up of atoms destroyed Atoms of same element look the same Chem reactions occur when atoms are rearranged Compounds formed by 2 or more atoms Describe the basic structure of the atom as protons, neutrons and ...
Chapter 10 Power Point - Biloxi Public Schools
... isotopes accounts for the average atomic mass. Average atomic mass - the average mass of the mixture of an element & its isotopes; only a whole number if radioactive or man-made; usually carried to 2 or 3 places past the decimal. The # of protons determines what the element is; the # of neutrons det ...
... isotopes accounts for the average atomic mass. Average atomic mass - the average mass of the mixture of an element & its isotopes; only a whole number if radioactive or man-made; usually carried to 2 or 3 places past the decimal. The # of protons determines what the element is; the # of neutrons det ...
Atomic Structure
... Different atoms have different ____________ and ____________ The differing properties of matter are due to the size, shape, and movement of ____________ Changes in matter result from changes in the ____________ of atoms and not the atoms themselves ...
... Different atoms have different ____________ and ____________ The differing properties of matter are due to the size, shape, and movement of ____________ Changes in matter result from changes in the ____________ of atoms and not the atoms themselves ...
DALTON`S ATOMIC THEORY - 1808: Publication of Dalton`s "A New
... LAW OF DEFINITE PROPORTIONS (also called the LAW OF CONSTANT COMPOSITION): All pure samples of a given compound contain the same proportion of elements by mass ...
... LAW OF DEFINITE PROPORTIONS (also called the LAW OF CONSTANT COMPOSITION): All pure samples of a given compound contain the same proportion of elements by mass ...
a) air c) milk f) beer
... Dalton’s Atomic Theory (in his own words): Atomic Theory – An explanation of the structure of matter in terms of different combinations of very small particles. ...
... Dalton’s Atomic Theory (in his own words): Atomic Theory – An explanation of the structure of matter in terms of different combinations of very small particles. ...
Test Review: Unit 1 - Ms. Hill`s Pre
... normal chemical and physical changes (Dalton didn’t describe/clarify normal circumstances, matter can be created and destroyed in nuclear reactions) b. Law of Definite Proportions: the fact that a chemical compound contain exactly the same elements in exactly the same proportions in exactly the same ...
... normal chemical and physical changes (Dalton didn’t describe/clarify normal circumstances, matter can be created and destroyed in nuclear reactions) b. Law of Definite Proportions: the fact that a chemical compound contain exactly the same elements in exactly the same proportions in exactly the same ...
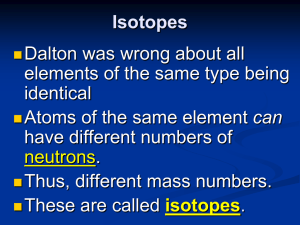
Isotopes
... Isotopes are atoms of the same element having different masses, due to varying numbers of neutrons. Isotope ...
... Isotopes are atoms of the same element having different masses, due to varying numbers of neutrons. Isotope ...
Atoms and their Structure
... and electricity, usually bend w/o breaking (malleable) • Almost all are solids at room temp. and have extremely high melting points ...
... and electricity, usually bend w/o breaking (malleable) • Almost all are solids at room temp. and have extremely high melting points ...
Chemical Formulas
... HC 2 H 3 O 2 (vinegar), NaHCO 3 (baking soda), NH 4 (ammonia), and C 6 H 12 O 6 (glucose). ...
... HC 2 H 3 O 2 (vinegar), NaHCO 3 (baking soda), NH 4 (ammonia), and C 6 H 12 O 6 (glucose). ...
Early Models of Atom
... Dalton: an English teacher who proposed that atoms are the smallest particles of matter. Model: 1. Each element is composed of indivisible particles called atoms 2. In an element, all of the atoms are identical. Atoms of different elements have different properties, such as mass. 3. In chemical reac ...
... Dalton: an English teacher who proposed that atoms are the smallest particles of matter. Model: 1. Each element is composed of indivisible particles called atoms 2. In an element, all of the atoms are identical. Atoms of different elements have different properties, such as mass. 3. In chemical reac ...
TEK 8.5D: Chemical Formulas
... HC 2 H 3 O 2 (vinegar), NaHCO 3 (baking soda), NH 4 (ammonia), and C 6 H 12 O 6 (glucose). ...
... HC 2 H 3 O 2 (vinegar), NaHCO 3 (baking soda), NH 4 (ammonia), and C 6 H 12 O 6 (glucose). ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.