Document
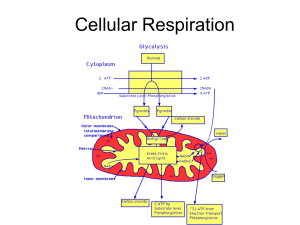
... Glucose is broken down with or without oxygen in the cytoplasm into pyruvate One Glucose is cleaved into two pyruvate Produces little energy Two ATP and Two NADH produced ...
... Glucose is broken down with or without oxygen in the cytoplasm into pyruvate One Glucose is cleaved into two pyruvate Produces little energy Two ATP and Two NADH produced ...
Chapter 6 ENZYME SUBSTRATE REACTANTS PRODUCTS
... 4. This term includes all the chemical reactions that allow cells to build and break down substances. Metabolism 5. This is the pocket in the enzyme into which the substrate bind. Active Site 6. Conditions such as extreme pH, temperature of salt cause enzymes to do this. Denature 7. This term descri ...
... 4. This term includes all the chemical reactions that allow cells to build and break down substances. Metabolism 5. This is the pocket in the enzyme into which the substrate bind. Active Site 6. Conditions such as extreme pH, temperature of salt cause enzymes to do this. Denature 7. This term descri ...
Lecture notes Chapter 27-28
... Within complex I, the NADH electrons are transferred to iron-sulfur (Fe-S) clusters and then to coenzyme (Q). FMNH2 + Q → QH2 + FMN The overall reaction sequence in complex I can be written as follows: NADH + H+ + Q → QH2 + NAD+ Complex II: Succinate Dehydrogenase: complex II is specifically used wh ...
... Within complex I, the NADH electrons are transferred to iron-sulfur (Fe-S) clusters and then to coenzyme (Q). FMNH2 + Q → QH2 + FMN The overall reaction sequence in complex I can be written as follows: NADH + H+ + Q → QH2 + NAD+ Complex II: Succinate Dehydrogenase: complex II is specifically used wh ...
Cellular Energy
... Occurs in the cytoplasm of cells • Glucose is converted into pyruvate producing a small amount of ATP. • (Remember glucose is an energy source.) • If there is not enough glucose for glycolysis, then lipids can be used. (ex. fats = energy storage) • This is what happens when we diet. ...
... Occurs in the cytoplasm of cells • Glucose is converted into pyruvate producing a small amount of ATP. • (Remember glucose is an energy source.) • If there is not enough glucose for glycolysis, then lipids can be used. (ex. fats = energy storage) • This is what happens when we diet. ...
“Photosynthesis and Respiration Concept Map” Use the terms below
... Typically a concept map goes from general or big ideas to smaller more specific or detailed ideas. Additionally, a connecting phrase describes the relationship between each of the terms. Use all of the terms below (each should be in a box or bubble) to create a concept map about photosynthesis and r ...
... Typically a concept map goes from general or big ideas to smaller more specific or detailed ideas. Additionally, a connecting phrase describes the relationship between each of the terms. Use all of the terms below (each should be in a box or bubble) to create a concept map about photosynthesis and r ...
No Slide Title
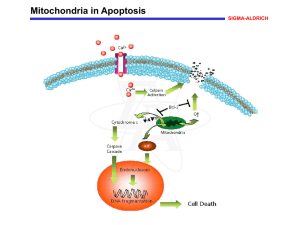
... Increases in cytosolic Ca2+ levels due to activation of ion channel-linked receptors, such as that for the excitatory amino acid neurotransmitter glutamic acid, can induce permeability transition (PT) of the mitochondrial membrane. PT constitutes the first rate-limiting event of the common pathway o ...
... Increases in cytosolic Ca2+ levels due to activation of ion channel-linked receptors, such as that for the excitatory amino acid neurotransmitter glutamic acid, can induce permeability transition (PT) of the mitochondrial membrane. PT constitutes the first rate-limiting event of the common pathway o ...
Slide 1
... The potential energy of this proton gradient is used to make ATP by chemiosmosis The concentration gradient drives H+ through ATP synthases producing ATP The ETC + chemiosmosis = oxidative phosphorylation Products: 34-38 ATP/glucose ...
... The potential energy of this proton gradient is used to make ATP by chemiosmosis The concentration gradient drives H+ through ATP synthases producing ATP The ETC + chemiosmosis = oxidative phosphorylation Products: 34-38 ATP/glucose ...
Respiration
... 21. Why do fats provide a little more than twice as many calories per gram as compared to carbohydrates or proteins? Hint: Think of the output of the Citric Acid Cycle. ...
... 21. Why do fats provide a little more than twice as many calories per gram as compared to carbohydrates or proteins? Hint: Think of the output of the Citric Acid Cycle. ...
Lecture Presentation to accompany Principles of Life
... • 6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play Important Roles in Biological Energy Metabolism • 6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy • 6.3 Carbohydrate Catabolism in the Absence of Oxygen Releases a Small Amount of Energy ...
... • 6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play Important Roles in Biological Energy Metabolism • 6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy • 6.3 Carbohydrate Catabolism in the Absence of Oxygen Releases a Small Amount of Energy ...
You Light Up My Life
... • All of the carbon molecules in pyruvate end up in carbon dioxide - released from cells • Coenzymes are reduced (they pick up electrons and hydrogen) - energized • One molecule of ATP is formed - just one! ...
... • All of the carbon molecules in pyruvate end up in carbon dioxide - released from cells • Coenzymes are reduced (they pick up electrons and hydrogen) - energized • One molecule of ATP is formed - just one! ...
The citric acid cycle • Also known as the Kreb`s cycle
... • Energy of succinyl CoA is transferred (conserved) to GTP • SUBSTRATE LEVEL PHOSPHORYLATION: group transfer reaction • ONLY step where ATP is directly formed • All other ATP is produced by oxidative phosphorylation Oxid. Phosphor. is the oxidation of reduced cofactors (NADH, FADH2), to form ATP fro ...
... • Energy of succinyl CoA is transferred (conserved) to GTP • SUBSTRATE LEVEL PHOSPHORYLATION: group transfer reaction • ONLY step where ATP is directly formed • All other ATP is produced by oxidative phosphorylation Oxid. Phosphor. is the oxidation of reduced cofactors (NADH, FADH2), to form ATP fro ...
Exam 1 Q2 Review Sheet
... used/made and NADH/FADH2 made for every step. (The following terms MUST be properly included: ETC, chemiosmosis, oxidative phosphorylation, electron carriers, heme, cytochrome c, ubiquinone, complex I, complex II, complex III, complex IV, mitochondria, NAD+, NADH, citrate, citrate synthase, hexokina ...
... used/made and NADH/FADH2 made for every step. (The following terms MUST be properly included: ETC, chemiosmosis, oxidative phosphorylation, electron carriers, heme, cytochrome c, ubiquinone, complex I, complex II, complex III, complex IV, mitochondria, NAD+, NADH, citrate, citrate synthase, hexokina ...
Lecture 8 - People Server at UNCW
... • Examine neural control of breathing • Respiratory centers in the brain • Peripheral input to respirator centers ...
... • Examine neural control of breathing • Respiratory centers in the brain • Peripheral input to respirator centers ...
Key Terms:
... How is respiration commonly regulated? Why might a cell want to slow down respiration? Lecture Outline: Anaerobic Metabolism recall that in glycolysis no oxygen required 2 ATP generated (net) per glucose but there's an NAD+/NADH problem! continuous running of glycolysis will use up all of your NAD ...
... How is respiration commonly regulated? Why might a cell want to slow down respiration? Lecture Outline: Anaerobic Metabolism recall that in glycolysis no oxygen required 2 ATP generated (net) per glucose but there's an NAD+/NADH problem! continuous running of glycolysis will use up all of your NAD ...
Classification and Nomenclature of Enzymes
... where “a” is the class, “b” is the subclass, “c” is the sub‐subclass, and “d” is the sub‐sub‐subclass. The “b” and “c” digits describe the reaction, while the “d” digit is used to distinguish between different enzymes of the same function based on the actual substrate in the reaction. • Exampl ...
... where “a” is the class, “b” is the subclass, “c” is the sub‐subclass, and “d” is the sub‐sub‐subclass. The “b” and “c” digits describe the reaction, while the “d” digit is used to distinguish between different enzymes of the same function based on the actual substrate in the reaction. • Exampl ...
CELLULAR RESPIRATION Fates of Pyruvate from glycolysis (2
... Metabolism—the sum of all biochemical reactions in an organism or cell. a) anabolic—synthesis of compounds; an example is photosynthesis b) catabolic—breakdown of compounds; an example is cellular respiration Metabolic pathways—are the steps (enzymes, substrates and products) used or followed to con ...
... Metabolism—the sum of all biochemical reactions in an organism or cell. a) anabolic—synthesis of compounds; an example is photosynthesis b) catabolic—breakdown of compounds; an example is cellular respiration Metabolic pathways—are the steps (enzymes, substrates and products) used or followed to con ...
Relationship between Photosynthesis and Cellular Respiration
... Stage II: Prep Stage (mitochondrial matrix) Stage III: Citric Acid Cycle (mitochondrial matrix) Stage IV: Electron Transport Chain oxidation- reduction reactions using NADH, FADH2 (mitochondrial cristae) ...
... Stage II: Prep Stage (mitochondrial matrix) Stage III: Citric Acid Cycle (mitochondrial matrix) Stage IV: Electron Transport Chain oxidation- reduction reactions using NADH, FADH2 (mitochondrial cristae) ...
File
... Electrons Lost = Electrons Gained Both sodium chloride and magnesium oxide are simple ionic compounds. ...
... Electrons Lost = Electrons Gained Both sodium chloride and magnesium oxide are simple ionic compounds. ...
Slide 1
... – a carboxyl group is removed and given off as _______, – the two-carbon compound remaining is oxidized while a molecule of NAD+ is reduced to __________, – coenzyme A joins with the two-carbon group to form acetyl coenzyme A, abbreviated as acetyl CoA, and – acetyl CoA enters the citric acid cycle. ...
... – a carboxyl group is removed and given off as _______, – the two-carbon compound remaining is oxidized while a molecule of NAD+ is reduced to __________, – coenzyme A joins with the two-carbon group to form acetyl coenzyme A, abbreviated as acetyl CoA, and – acetyl CoA enters the citric acid cycle. ...
electron transport chain
... glucose NADH electron transport chain proton-motive force ATP • About 34% of the energy in a glucose molecule is transferred to ATP during cellular respiration, making about 32 ATP • There are several reasons why the number of ATP is not known exactly © 2011 Pearson Education, Inc. ...
... glucose NADH electron transport chain proton-motive force ATP • About 34% of the energy in a glucose molecule is transferred to ATP during cellular respiration, making about 32 ATP • There are several reasons why the number of ATP is not known exactly © 2011 Pearson Education, Inc. ...
Electron transport chain
An electron transport chain (ETC) is a series of compounds that transfer electrons from electron donors to electron acceptors via redox reactions, and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. This creates an electrochemical proton gradient that drives ATP synthesis, or the generation of chemical energy in the form of adenosine triphosphate (ATP). The final acceptor of electrons in the electron transport chain is molecular oxygen.Electron transport chains are used for extracting energy via redox reactions from sunlight in photosynthesis or, such as in the case of the oxidation of sugars, cellular respiration. In eukaryotes, an important electron transport chain is found in the inner mitochondrial membrane where it serves as the site of oxidative phosphorylation through the use of ATP synthase. It is also found in the thylakoid membrane of the chloroplast in photosynthetic eukaryotes. In bacteria, the electron transport chain is located in their cell membrane.In chloroplasts, light drives the conversion of water to oxygen and NADP+ to NADPH with transfer of H+ ions across chloroplast membranes. In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that are required to generate the proton gradient. Electron transport chains are major sites of premature electron leakage to oxygen, generating superoxide and potentially resulting in increased oxidative stress.