![Atomic Structure [PowerPoint]](http://s1.studyres.com/store/data/000122096_1-1d100da6540d2f26db122fc51f672fe5-300x300.png)
Name
... • An atom is the smallest particle of an element that retains the chemical properties of that element. • The nucleus is a very small region located at the center of an atom. • The nucleus is made up of at least one positively charged particle called a proton and usually one or more neutral particles ...
... • An atom is the smallest particle of an element that retains the chemical properties of that element. • The nucleus is a very small region located at the center of an atom. • The nucleus is made up of at least one positively charged particle called a proton and usually one or more neutral particles ...
Structure of Atoms/Periodic Table Review 1. Shade in location of the
... 20. Why are atoms neutral? They have the _________ __________ of protons and neutrons. 22. What are the limitations of the Bohr model? ____________ do not orbit the nucleus like planets orbit the sun. The ___________ does not represent the actual size of an atom. 23. What determines an element’s ide ...
... 20. Why are atoms neutral? They have the _________ __________ of protons and neutrons. 22. What are the limitations of the Bohr model? ____________ do not orbit the nucleus like planets orbit the sun. The ___________ does not represent the actual size of an atom. 23. What determines an element’s ide ...
Power point on the Periodic Table
... Proton: in the nucleus, symbol “p”, positive charge, much larger mass than an electron ...
... Proton: in the nucleus, symbol “p”, positive charge, much larger mass than an electron ...
Chapter 2—Atoms, Molecules, and Ions
... • In 1911, Rutherford proved the existence of the proton with his nowfamous gold-foil experiment • He shot α-particles at the foil…most passed through, but some were reflected back at the fluorescent ...
... • In 1911, Rutherford proved the existence of the proton with his nowfamous gold-foil experiment • He shot α-particles at the foil…most passed through, but some were reflected back at the fluorescent ...
Unit 3 Study Guide
... Unit 2 Review-Atomic Theory 1. According to Dalton’s theory atoms can’t be created or destroyed. Is this still true today? Explain. No, it is not true. In Dalton’s time technology for splitting atoms was not yet developed. 2. According to Dalton’s theory all atoms of the same element are identical i ...
... Unit 2 Review-Atomic Theory 1. According to Dalton’s theory atoms can’t be created or destroyed. Is this still true today? Explain. No, it is not true. In Dalton’s time technology for splitting atoms was not yet developed. 2. According to Dalton’s theory all atoms of the same element are identical i ...
File - Mr. Gittermann
... with no charge and is located in the nucleus of the atom • Electrons: Subatomic particle with a negative charge found in a certain region of space around the nucleus called the electron cloud; kept close to the atom due to the attraction between the opposite charges of the electron and proton ...
... with no charge and is located in the nucleus of the atom • Electrons: Subatomic particle with a negative charge found in a certain region of space around the nucleus called the electron cloud; kept close to the atom due to the attraction between the opposite charges of the electron and proton ...
Chapter 5 Section 1 - Ms. Halbohm`s Classroom
... 2. What determines the length of each period in the periodic table? 3. What is the relationship between the electron configuration of an element and the period in which that element appears in the periodic table? 4. What information is provided by the specific block location of an element? Identify, ...
... 2. What determines the length of each period in the periodic table? 3. What is the relationship between the electron configuration of an element and the period in which that element appears in the periodic table? 4. What information is provided by the specific block location of an element? Identify, ...
Isotopes, Ions Worksheet
... b) Do different isotopes have different half-lifes (t ½ )? YES Different isotopes have a different neutron number which results in different half-life 21. List THREE Nuclear Applications 1. _______________________ 2. ______________________ 3. _______________________ 22. Why is it important to know t ...
... b) Do different isotopes have different half-lifes (t ½ )? YES Different isotopes have a different neutron number which results in different half-life 21. List THREE Nuclear Applications 1. _______________________ 2. ______________________ 3. _______________________ 22. Why is it important to know t ...
chapter_four
... found outside the nucleus in regions called orbitals Protons are positively charged and found in the nucleus of an atom with neutrons, which have no charge There are even smaller particles but we do not study ...
... found outside the nucleus in regions called orbitals Protons are positively charged and found in the nucleus of an atom with neutrons, which have no charge There are even smaller particles but we do not study ...
The Periodic Table OL Page 1 of 2 G. Galvin Name: Periodic Table
... 1. Mendeleev: Arranged the elements in order of increasing weight. Defn: Mendeleev’s Periodic Law: When elements are arranged in order of increasing atomic weight, the properties of the elements recur periodically, i.e. the properties displayed by the element are repeated at regular intervals in oth ...
... 1. Mendeleev: Arranged the elements in order of increasing weight. Defn: Mendeleev’s Periodic Law: When elements are arranged in order of increasing atomic weight, the properties of the elements recur periodically, i.e. the properties displayed by the element are repeated at regular intervals in oth ...
Document
... An industrially important element contains 26 electrons and rusts in the presence of air and moisture. Identify the element. ...
... An industrially important element contains 26 electrons and rusts in the presence of air and moisture. Identify the element. ...
Isotopes and Shell Diagrams
... Since isotopes of an element have different numbers of neutrons, they also have different mass numbers and different atomic masses. ...
... Since isotopes of an element have different numbers of neutrons, they also have different mass numbers and different atomic masses. ...
Atomic Theory and Structure
... If it’s so small, how was it discovered? • Not long after Greece finally defeated Persia, a Greek philosopher named Democritus did some thinking – If you cut something in half, and then cut that in half, and keep going, you’ll end up with something you can’t cut ...
... If it’s so small, how was it discovered? • Not long after Greece finally defeated Persia, a Greek philosopher named Democritus did some thinking – If you cut something in half, and then cut that in half, and keep going, you’ll end up with something you can’t cut ...
Atomic Size
... • The apparent discontinuities in this diagram reflect the difficulty of comparing the radii of atoms of metallic and nonmetallic bonding types. Radii of the noble gas elements are estimates from those of nearby elements. ...
... • The apparent discontinuities in this diagram reflect the difficulty of comparing the radii of atoms of metallic and nonmetallic bonding types. Radii of the noble gas elements are estimates from those of nearby elements. ...
Isotope Practice Worksheet
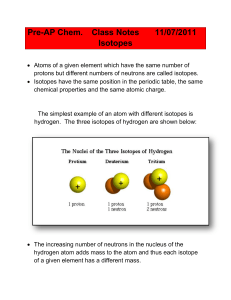
... Atoms of a given element which have the same number of protons but different numbers of neutrons are called isotopes. Isotopes have the same position in the periodic table, the same chemical properties and the same atomic charge. ...
... Atoms of a given element which have the same number of protons but different numbers of neutrons are called isotopes. Isotopes have the same position in the periodic table, the same chemical properties and the same atomic charge. ...
Atoms, Molecules and Ions
... Dalton’s Atomic Theory (1808) (Listed on p 203) 1. Elements are composed of extremely small particles called atoms. 2. All atoms of a given element are identical, having the same size, mass and chemical properties. 3. The atoms of one element are different from the atoms of all other elements. 4. C ...
... Dalton’s Atomic Theory (1808) (Listed on p 203) 1. Elements are composed of extremely small particles called atoms. 2. All atoms of a given element are identical, having the same size, mass and chemical properties. 3. The atoms of one element are different from the atoms of all other elements. 4. C ...
Atomic Structure and the Composition of Matter
... mass and are ~1800 times more massive than the electron. Both nuclear particles are composed of quarks, smaller fundamental particles. • Protons have unit positive charge (+1), while electrons have unit negative charge (-1). Neutrons ...
... mass and are ~1800 times more massive than the electron. Both nuclear particles are composed of quarks, smaller fundamental particles. • Protons have unit positive charge (+1), while electrons have unit negative charge (-1). Neutrons ...
General CHemistry Unit 2 Homework Notes
... A neutron has no charge and a relative mass of one. TOPIC TWO: COMPOUNDS & BONDING (PAGE 2) Subscripts in a chemical formula represent the relative number of each type of atom. The subscript always follows the symbol for the element. Example: In a water molecule, H2O, there are 2 hydrogen atoms and ...
... A neutron has no charge and a relative mass of one. TOPIC TWO: COMPOUNDS & BONDING (PAGE 2) Subscripts in a chemical formula represent the relative number of each type of atom. The subscript always follows the symbol for the element. Example: In a water molecule, H2O, there are 2 hydrogen atoms and ...
Unit 3 Note Outline
... Atoms that have the same number of Most elements have more than one isotope - Hydrogen has Hydrogen-1 has Hydrogen-2 has Hydrogen-3 has ...
... Atoms that have the same number of Most elements have more than one isotope - Hydrogen has Hydrogen-1 has Hydrogen-2 has Hydrogen-3 has ...
File
... Equal to the number of protons plus the number of neutrons in an atom of the element Each element has many possible mass numbers, but the most commonly occurring mass number is determined by rounding the atomic mass to the nearest whole number ...
... Equal to the number of protons plus the number of neutrons in an atom of the element Each element has many possible mass numbers, but the most commonly occurring mass number is determined by rounding the atomic mass to the nearest whole number ...
PS 2.2
... the weighted average of the masses of the naturally occurring isotopes of an element. The atomic mass of an element can be found on the periodic table. Since it is an average, it is usually not a whole number. ...
... the weighted average of the masses of the naturally occurring isotopes of an element. The atomic mass of an element can be found on the periodic table. Since it is an average, it is usually not a whole number. ...
C1.1 The fundamental ideas in chemistry
... When elements react, their atoms join with other atoms to form compounds. This involves giving, taking or sharing electrons to form ions or molecules. Compounds formed from metals and non-metals consist of ions. Compounds formed from nonmetals consist of molecules. In molecules the atoms are held to ...
... When elements react, their atoms join with other atoms to form compounds. This involves giving, taking or sharing electrons to form ions or molecules. Compounds formed from metals and non-metals consist of ions. Compounds formed from nonmetals consist of molecules. In molecules the atoms are held to ...
Atomic Structure: SOL Review #1 Name: Historical Developments 1
... The electrons are not “singly before pairing.” Electrons repel each other, so they do want not pair until there is no more “space” left in the sublevel. Periodic Table and Periodic Trends 27) Which elements would have similar properties to Na? ...
... The electrons are not “singly before pairing.” Electrons repel each other, so they do want not pair until there is no more “space” left in the sublevel. Periodic Table and Periodic Trends 27) Which elements would have similar properties to Na? ...