File
... 32. Give an example of a compound. H2O 33. What is a molecule? An element with more than one atom attached to it 34. Give an example of a molecule. O₂- air we breathe O₃- ozone layer 35. As you go from left to right on the periodic table, describe the changes that occur to element's atomic structure ...
... 32. Give an example of a compound. H2O 33. What is a molecule? An element with more than one atom attached to it 34. Give an example of a molecule. O₂- air we breathe O₃- ozone layer 35. As you go from left to right on the periodic table, describe the changes that occur to element's atomic structure ...
Radioactive Isotopes and Nuclear Equations
... b. Identify the radioactive isotope that decays to produce a neutron and phosphorus-30 when bombarded with an alpha particle. ...
... b. Identify the radioactive isotope that decays to produce a neutron and phosphorus-30 when bombarded with an alpha particle. ...
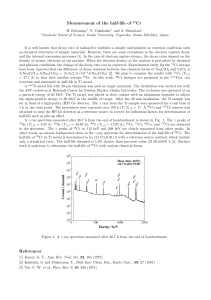
Measurement of the half-life of
... density of atomic electrons at the nucleus. When the electron density at the nucleus is perturbed by chemical and physical conditions, the change of the decay rate can be expected. Experimental study for the 51 Cr isotope have been reported that the difference of decay constant between two chemical f ...
... density of atomic electrons at the nucleus. When the electron density at the nucleus is perturbed by chemical and physical conditions, the change of the decay rate can be expected. Experimental study for the 51 Cr isotope have been reported that the difference of decay constant between two chemical f ...
Chemistry and elements 1. The rows of the periodic table are called
... 1. The rows of the periodic table are called: a. classes b. periods c. groups d. families 2. As you move from left to right across the periodic table: a. atomic number decreases b. atomic number increases c. The elements become darker in color d. The elements become lighter in color 4. Where would y ...
... 1. The rows of the periodic table are called: a. classes b. periods c. groups d. families 2. As you move from left to right across the periodic table: a. atomic number decreases b. atomic number increases c. The elements become darker in color d. The elements become lighter in color 4. Where would y ...
Chapter 10 Power Point - Biloxi Public Schools
... atomic masses & discovered a repeating pattern of properties or characteristics. ***There were some gaps in masses so he placed question marks in their spots. Later, elements were discovered to fill in these gaps. His predictions about elements, their masses & properties proved to be true.*** Henry ...
... atomic masses & discovered a repeating pattern of properties or characteristics. ***There were some gaps in masses so he placed question marks in their spots. Later, elements were discovered to fill in these gaps. His predictions about elements, their masses & properties proved to be true.*** Henry ...
Big History Chemistry Study Guide File
... of _____ and _____. These isotopes have _____ neutrons or ____ neutrons. 7. In nuclear ________________, small atoms combine to make larger atoms, losing a tiny bit of mass and releasing energy in the process. 8. In nuclear _____________, radioactive elements such as ________________ break apart int ...
... of _____ and _____. These isotopes have _____ neutrons or ____ neutrons. 7. In nuclear ________________, small atoms combine to make larger atoms, losing a tiny bit of mass and releasing energy in the process. 8. In nuclear _____________, radioactive elements such as ________________ break apart int ...
Isotopes and Atomic Mass
... Insights into Student Use • In college interviews, students wanted to select other common elements such as gold; investigation into other elements could be incorporated as part of an activity. • On the Mixtures screen, students attempted to match Nature’s Mix using My Mix view. This is not possible ...
... Insights into Student Use • In college interviews, students wanted to select other common elements such as gold; investigation into other elements could be incorporated as part of an activity. • On the Mixtures screen, students attempted to match Nature’s Mix using My Mix view. This is not possible ...
Article 2: Key Concepts and Vocabulary
... most of the electricity is sent on transmission lines to provide consumers with power for refrigerators, lights, computers, and many other devices. Although amounts of energy are expressed in many different units, depending on the country and the context, the scientific community uses a unified syst ...
... most of the electricity is sent on transmission lines to provide consumers with power for refrigerators, lights, computers, and many other devices. Although amounts of energy are expressed in many different units, depending on the country and the context, the scientific community uses a unified syst ...
2.9 Use the helium-4 isotope to define atomic number and mass
... 2.23 Elements whose names end with ium are usually metals; sodium is one example. Identify a nonmetal whose name also ends with ium. ...
... 2.23 Elements whose names end with ium are usually metals; sodium is one example. Identify a nonmetal whose name also ends with ium. ...
The Periodic Table
... Mass number is the count of nucleons in an isotope and atomic mass is the measure of the average mass of an atom including the relative abundance of its element’s isotopes. ...
... Mass number is the count of nucleons in an isotope and atomic mass is the measure of the average mass of an atom including the relative abundance of its element’s isotopes. ...
History of the Atomic Model
... Isotopes: Atoms of the same element that have different numbers of neutrons – Due to isotopes, mass #s are not round #s. – E.g. Li (6.9) is made up of both 6Li and 7Li. – Often, at least one isotope is unstable.It breaks down, releasing radioactivity.These types of isotopes are called radioisotopes ...
... Isotopes: Atoms of the same element that have different numbers of neutrons – Due to isotopes, mass #s are not round #s. – E.g. Li (6.9) is made up of both 6Li and 7Li. – Often, at least one isotope is unstable.It breaks down, releasing radioactivity.These types of isotopes are called radioisotopes ...
Isotopes
... Soddy won the Nobel Prize in Chemistry in 1921 for his work with isotopes and radioactive materials. ...
... Soddy won the Nobel Prize in Chemistry in 1921 for his work with isotopes and radioactive materials. ...
Periodic Table Quiz
... They have the ability to be pulled into wires. They have the ability to be hammered into sheets. They have the ability to be liquid at room temperature. They have the ability to conduct electricity only under certain conditions. ...
... They have the ability to be pulled into wires. They have the ability to be hammered into sheets. They have the ability to be liquid at room temperature. They have the ability to conduct electricity only under certain conditions. ...
Chemistry Notes (pg. # 1)
... - Also, how did they determine that there is a ____________ and an electron “__________”? Evidence for the Negative Electron: ...
... - Also, how did they determine that there is a ____________ and an electron “__________”? Evidence for the Negative Electron: ...
a worksheet on C1.1
... atom is called an element. There are about 100 different elements. Elements are shown in the periodic table. The groups contain elements with similar properties. Candidates should understand where metals and non-metals appear in the periodic table. b) Atoms of each element are represented by a chemi ...
... atom is called an element. There are about 100 different elements. Elements are shown in the periodic table. The groups contain elements with similar properties. Candidates should understand where metals and non-metals appear in the periodic table. b) Atoms of each element are represented by a chemi ...
CHEMISTRY The Molecular Science
... • An element is composed of tiny particles called atoms. All atoms of a given element show the same chemical properties. • Atoms of different elements have different properties. • Compounds are formed when atoms of two or more elements combine. In a given compound, the relative number of atoms of ea ...
... • An element is composed of tiny particles called atoms. All atoms of a given element show the same chemical properties. • Atoms of different elements have different properties. • Compounds are formed when atoms of two or more elements combine. In a given compound, the relative number of atoms of ea ...
What does the Periodic Table tell us?
... are vertical (up and down) elements in the same group/family ________________________________ there are ____of them on the periodic table o Ex) Li, Na, K all have a similar ____________________ with water (H2O). They all create an explosion that releases hydrogen gas (H2). Therefore, they are ...
... are vertical (up and down) elements in the same group/family ________________________________ there are ____of them on the periodic table o Ex) Li, Na, K all have a similar ____________________ with water (H2O). They all create an explosion that releases hydrogen gas (H2). Therefore, they are ...
Exam Review/SLO 1 Topics Mixtures Have two or more different
... Depend on type of matter present (density, melting point, boiling point, etc.) Do not depend on how much matter is present Physical Properties Properties which can be observed without a change in identity (chemical make-up) Examples: boiling point, melting point, density Density = mass / volume (alt ...
... Depend on type of matter present (density, melting point, boiling point, etc.) Do not depend on how much matter is present Physical Properties Properties which can be observed without a change in identity (chemical make-up) Examples: boiling point, melting point, density Density = mass / volume (alt ...
Atomic Structure
... ________ The Alkali Earth with the smallest atomic radius. ________ Element number 14 (element with atomic number 14). ________ The second period noble gas. ________ The first metal in group 1. ________ An element that reacts like chlorine, but has a smaller atomic radius. ________ A third period el ...
... ________ The Alkali Earth with the smallest atomic radius. ________ Element number 14 (element with atomic number 14). ________ The second period noble gas. ________ The first metal in group 1. ________ An element that reacts like chlorine, but has a smaller atomic radius. ________ A third period el ...
Periodic Trends
... atoms. Let’s take Na and Cl for example. They are both on n=3. But Cl has 17 protons pulling on n=3 and Na only has 11 protons pulling on n=3. Cl is smaller because it’s nuclear charge is greater. That fact is very important for the trends. Once we can see that the nonmetals tend to be smaller with ...
... atoms. Let’s take Na and Cl for example. They are both on n=3. But Cl has 17 protons pulling on n=3 and Na only has 11 protons pulling on n=3. Cl is smaller because it’s nuclear charge is greater. That fact is very important for the trends. Once we can see that the nonmetals tend to be smaller with ...
Study guide for percent abundance chapter 4 spaced out
... 15.Which statement is correct for the emission spectrum of the hydrogen atom? a. The lines converge at lower energies b. The lines are produced when electrons move from lower to higher energy levels c. The lines in the visible region involve electron transition into the energy level closest to the ...
... 15.Which statement is correct for the emission spectrum of the hydrogen atom? a. The lines converge at lower energies b. The lines are produced when electrons move from lower to higher energy levels c. The lines in the visible region involve electron transition into the energy level closest to the ...
Atomic Timeline - Ms Brown`s Chemistry Page
... • John Dalton formulated the Atomic Theory which states that: atoms of an element are different than atoms of other elements; atoms of one element are the same; that atoms of different elements can be combined; that atoms cannot be divided or separated; and that elements are made of tiny particles c ...
... • John Dalton formulated the Atomic Theory which states that: atoms of an element are different than atoms of other elements; atoms of one element are the same; that atoms of different elements can be combined; that atoms cannot be divided or separated; and that elements are made of tiny particles c ...