Reading Assignment Worksheet on Atoms - District 196 e
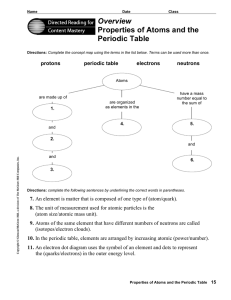
... Directions: Complete the concept map using the terms in the list below. Terms can be used more than once. ...
... Directions: Complete the concept map using the terms in the list below. Terms can be used more than once. ...
The History of the Modern Periodic Table
... In 1913, through his work with X-rays, he determined the actual nuclear charge (atomic number) of the elements*. He rearranged the elements in order of increasing atomic number. *“There is in the atom a fundamental quantity which increases by regular steps as we pass from each element to the next. T ...
... In 1913, through his work with X-rays, he determined the actual nuclear charge (atomic number) of the elements*. He rearranged the elements in order of increasing atomic number. *“There is in the atom a fundamental quantity which increases by regular steps as we pass from each element to the next. T ...
Chapter 2 Lect. 1
... 1. Becquerel (1896) discovered that uranium produced an image on photographic film in the absence of light 2. This spontaneous emission of radiation was called radioactivity 3. Three types of radioactive emission were eventually discovered a. Gamma rays (g) = high energy light wave b. Beta particles ...
... 1. Becquerel (1896) discovered that uranium produced an image on photographic film in the absence of light 2. This spontaneous emission of radiation was called radioactivity 3. Three types of radioactive emission were eventually discovered a. Gamma rays (g) = high energy light wave b. Beta particles ...
Nuclear Chemistry Worksheet
... The quantum mechanical model is based on quantum theory, which says matter also has properties associated with waves. According to quantum theory, it’s impossible to know the exact position and momentum of an electron at the same time. This is known as the Uncertainty Principle. The quantum mechanic ...
... The quantum mechanical model is based on quantum theory, which says matter also has properties associated with waves. According to quantum theory, it’s impossible to know the exact position and momentum of an electron at the same time. This is known as the Uncertainty Principle. The quantum mechanic ...
Bohr Models and Lewis Dot Structures
... • Periods go from left to • Groups go up and down right ...
... • Periods go from left to • Groups go up and down right ...
Understanding Atomic Structure of an Element
... • Electron Shells/Orbitals – An electron has only 1/1837th the mass of an proton or neutron – Electrons move around on specific patterns outside of the nucleus (the majority of the empty space in an atom) ...
... • Electron Shells/Orbitals – An electron has only 1/1837th the mass of an proton or neutron – Electrons move around on specific patterns outside of the nucleus (the majority of the empty space in an atom) ...
Atoms and the Periodic Table
... The elements are arranged on the periodic table by the number of protons and then grouped by other properties, such as: ...
... The elements are arranged on the periodic table by the number of protons and then grouped by other properties, such as: ...
Basics of Chemistry
... chemical behavior of an atom depends on number of electrons in its outermost shell ...
... chemical behavior of an atom depends on number of electrons in its outermost shell ...
MORE ABOUT PROTONS, NEUTRONS AND ELECTRONS
... If you take a good look at the Periodic table you will find that the elements all have their mass (weight) recorded in a.m.u. The figure used is the RELATIVE ATOMIC MASS. A definition of this figure is - the weighted mean of naturally occuring atoms of that element. It is very important to refer to ...
... If you take a good look at the Periodic table you will find that the elements all have their mass (weight) recorded in a.m.u. The figure used is the RELATIVE ATOMIC MASS. A definition of this figure is - the weighted mean of naturally occuring atoms of that element. It is very important to refer to ...
Unit 2 Exam Review: Matter and its Properties This review does not
... 21. Which elements are designated as noble gases? What is the most significant property of these elements? 22. This group of the periodic table is highly reactive and elements are soft enough to cut with a knife… 23. This group of the periodic table contains metals that exist in elemental form…; ...
... 21. Which elements are designated as noble gases? What is the most significant property of these elements? 22. This group of the periodic table is highly reactive and elements are soft enough to cut with a knife… 23. This group of the periodic table contains metals that exist in elemental form…; ...
Chapter 2choutline - Madison County Schools
... of an element. Because atoms have no overall electrical charge, an atom must have as many ____________________as there are protons in its nucleus. Therefore, the atomic number also tells the number of electrons in a ___________________atom. (chlorine has ______ electrons) The sum of the ____________ ...
... of an element. Because atoms have no overall electrical charge, an atom must have as many ____________________as there are protons in its nucleus. Therefore, the atomic number also tells the number of electrons in a ___________________atom. (chlorine has ______ electrons) The sum of the ____________ ...
Unit 4 Study Guide (Test on Friday 3/10) ANSWER
... electrons in the first energy level and 4 electrons in the second energy level. The only number of particles that changes is neutrons!!! Electron and proton numbers stay the same with each isotope! ...
... electrons in the first energy level and 4 electrons in the second energy level. The only number of particles that changes is neutrons!!! Electron and proton numbers stay the same with each isotope! ...
Unit 2 Outline
... The Periodic Law states that when elements are arranged in order of increasing atomic numbers, their physical and chemical properties show a periodic pattern. The names of groups and periods on the periodic chart are alkali metals, alkaline earth metals, transition metals, halogens, noble gases, ...
... The Periodic Law states that when elements are arranged in order of increasing atomic numbers, their physical and chemical properties show a periodic pattern. The names of groups and periods on the periodic chart are alkali metals, alkaline earth metals, transition metals, halogens, noble gases, ...
The periodic table is the most significant tool that chemist use for
... vigorously and stimulating much new work in chemistry. His insistence that elements with similar characteristics be listed in the same families forced him to leave several blank spaces in his table. Mendeleev boldly predicted their existence and properties. ...
... vigorously and stimulating much new work in chemistry. His insistence that elements with similar characteristics be listed in the same families forced him to leave several blank spaces in his table. Mendeleev boldly predicted their existence and properties. ...
Element: a pure, simple substance that can`t be broken down into
... What is the smallest unit of matter that we can find everywhere, even in tuna fish? What charge do electrons have? What are elements? Who organized the atomic elements? What do we call a horizontal row on the periodic table? What do we call the vertical columns on the periodic table? The number of p ...
... What is the smallest unit of matter that we can find everywhere, even in tuna fish? What charge do electrons have? What are elements? Who organized the atomic elements? What do we call a horizontal row on the periodic table? What do we call the vertical columns on the periodic table? The number of p ...
Unit 2: All Biology is Chemistry
... Isotopes are atoms of the same element that have different numbers of neutrons. – therefore they will have different mass numbers – this is the reason for the average atomic mass in the periodic table Click here to compare these twoare atoms. These two atoms both carbon atoms. But the atom on the le ...
... Isotopes are atoms of the same element that have different numbers of neutrons. – therefore they will have different mass numbers – this is the reason for the average atomic mass in the periodic table Click here to compare these twoare atoms. These two atoms both carbon atoms. But the atom on the le ...
Honors review- ch. 4 Element Symbol Atomic # Atomic mass
... each have a relative mass of 1 amu, so the average mass should not be a fraction of a number. 2. If two atoms have the same number of protons then they are the same element. Atoms of the same element with a different mass are called isotopes. 3. If two elements have the same mass, but different prot ...
... each have a relative mass of 1 amu, so the average mass should not be a fraction of a number. 2. If two atoms have the same number of protons then they are the same element. Atoms of the same element with a different mass are called isotopes. 3. If two elements have the same mass, but different prot ...
Chapter 5 “Atomic Structure and the Periodic table”
... 2)Atoms of the same element are identical. Atoms of any one element are different from those of any other element. 3)Atoms of different elements combine in simple whole-number ratios to form chemical compounds 4)In chemical reactions, atoms are combined, separated, or rearranged – but never changed ...
... 2)Atoms of the same element are identical. Atoms of any one element are different from those of any other element. 3)Atoms of different elements combine in simple whole-number ratios to form chemical compounds 4)In chemical reactions, atoms are combined, separated, or rearranged – but never changed ...
Chemistry - Spokane Public Schools
... The 1st energy level closest to the nucleus can have up to, but no more than 2 electrons. The 2nd energy level can have up to, but no more than 8 electrons. The 3rd energy level can have up to, but no more than 8 electrons. The 4th level farthest out can have up to, but no more than 18. (pg. 336) 15 ...
... The 1st energy level closest to the nucleus can have up to, but no more than 2 electrons. The 2nd energy level can have up to, but no more than 8 electrons. The 3rd energy level can have up to, but no more than 8 electrons. The 4th level farthest out can have up to, but no more than 18. (pg. 336) 15 ...
DALTON`S ATOMIC THEORY - 1808: Publication of Dalton`s "A New
... Example: Helium has an atomic number of 2. Every helium atom has two protons in its nucleus. - MASS NUMBER: The number of protons PLUS the number of neutrons in the atomic nucleus, Atoms of the same element may have DIFFERENT mass numbers. - ISOTOPES: are atoms of the same element with different mas ...
... Example: Helium has an atomic number of 2. Every helium atom has two protons in its nucleus. - MASS NUMBER: The number of protons PLUS the number of neutrons in the atomic nucleus, Atoms of the same element may have DIFFERENT mass numbers. - ISOTOPES: are atoms of the same element with different mas ...
Chapter 3, Section 1 Inside an Atom
... number of protons. The atomic number is equal to the number of protons in that element. The atomic number is a unique identifying characteristic. ...
... number of protons. The atomic number is equal to the number of protons in that element. The atomic number is a unique identifying characteristic. ...
Unit2StudyGuide
... Which two particles have the same mass? What particles make up the nucleus? What particles are NOT in the nucleus? What particles make up the atomic mass? What particles have insignificant mass, but take up most of the space of an atom? Atoms of the same element but have a different mass are _______ ...
... Which two particles have the same mass? What particles make up the nucleus? What particles are NOT in the nucleus? What particles make up the atomic mass? What particles have insignificant mass, but take up most of the space of an atom? Atoms of the same element but have a different mass are _______ ...
Chemistry Timeline
... Joseph John Thomson Polonium) Four elements Invented a good atomic theory (list all points) Matter is made up of “atomos” For each person include in a TYPED paragraph: 1. The name of the scientist with birth and death dates (as known). In other words, WHEN. 2. A complete explanation of their ...
... Joseph John Thomson Polonium) Four elements Invented a good atomic theory (list all points) Matter is made up of “atomos” For each person include in a TYPED paragraph: 1. The name of the scientist with birth and death dates (as known). In other words, WHEN. 2. A complete explanation of their ...
CHAPTER 4 ATOMIC STRUCTURE
... When an atom does not have the same ________of neutrons Same atomic number but different mass #’s Ex. Oxygen-16, 17, and 18 All oxygen atoms have 8 protons, but some have 9 or 10 neutrons • Ques. 1-7 pg. 112 ...
... When an atom does not have the same ________of neutrons Same atomic number but different mass #’s Ex. Oxygen-16, 17, and 18 All oxygen atoms have 8 protons, but some have 9 or 10 neutrons • Ques. 1-7 pg. 112 ...























