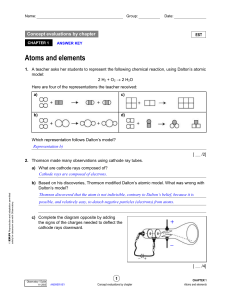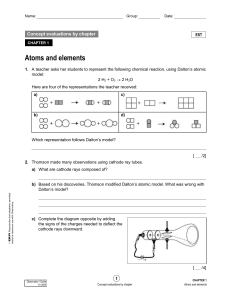
11129_evl_ch1_ste_eleve (3)
... is used extensively today in cellphone batteries and will also be used, in the near future, in batteries for hybrid or electric cars. In 2008, one battery out of every four sold in the world was manufactured in China. China therefore has a certain economic interest in Tibet. Mining the resource is r ...
... is used extensively today in cellphone batteries and will also be used, in the near future, in batteries for hybrid or electric cars. In 2008, one battery out of every four sold in the world was manufactured in China. China therefore has a certain economic interest in Tibet. Mining the resource is r ...
Trends in the Periodic Table
... Name: _________________________________ Period: ____ Date: ___ / ___ / ___ ...
... Name: _________________________________ Period: ____ Date: ___ / ___ / ___ ...
File
... Periodic Table and Ion Formation • Atoms gain and lose electrons to form bonds. When atoms become electrically charged particles, they are called ions. Metals lose electrons and become positive ions (called cations). Some metals (multivalent) lose electrons in different ways. For example, i ...
... Periodic Table and Ion Formation • Atoms gain and lose electrons to form bonds. When atoms become electrically charged particles, they are called ions. Metals lose electrons and become positive ions (called cations). Some metals (multivalent) lose electrons in different ways. For example, i ...
Atoms, Electrons and Periodicity test - A
... Complete the electronic configuration of a bromine atom. 1s22s22p63s23p6 ................................................................................................ ...
... Complete the electronic configuration of a bromine atom. 1s22s22p63s23p6 ................................................................................................ ...
Chapter 4 Atomic Structure
... Law of Octaves: noted that after interval of eight elements, similar physical/chemical properties reappeared. Newlands was the first to formulate the concept of periodicity (repeating patterns) in the properties of the chemical elements. ...
... Law of Octaves: noted that after interval of eight elements, similar physical/chemical properties reappeared. Newlands was the first to formulate the concept of periodicity (repeating patterns) in the properties of the chemical elements. ...
Groups of the Periodic Table
... 2. Work out which group the element is in and draw that number of electrons in the outer circle – with eight for Group 0 elements – except helium. 3. Fill the other circles with as many electrons as needed. Remember – two in the first circle, and eight in the second and third circles. 4. Finally, ch ...
... 2. Work out which group the element is in and draw that number of electrons in the outer circle – with eight for Group 0 elements – except helium. 3. Fill the other circles with as many electrons as needed. Remember – two in the first circle, and eight in the second and third circles. 4. Finally, ch ...
Science 9 - Ms. J Reed
... To determine the number of electrons in an ion you need to subtract the charge to the atomic number ◦ EX: Calcium has an atomic number of 20 and a 2+ charge.......so, its ion has 20 - 2 = 18 electrons ◦ EX: Fluorine has an atomic number of 9 and a 1charge.......so, its ion has 9 – (-1) = 10 electron ...
... To determine the number of electrons in an ion you need to subtract the charge to the atomic number ◦ EX: Calcium has an atomic number of 20 and a 2+ charge.......so, its ion has 20 - 2 = 18 electrons ◦ EX: Fluorine has an atomic number of 9 and a 1charge.......so, its ion has 9 – (-1) = 10 electron ...
valence electrons
... • Consists of the elements symbol, which represents the innermost electrons too, and the valence electrons surrounding it. • Example: Lithium has 3 electrons but only 1 valence----Li • The number of valence electrons determines how many and what this atom (or ion) can bond to in order to make a mole ...
... • Consists of the elements symbol, which represents the innermost electrons too, and the valence electrons surrounding it. • Example: Lithium has 3 electrons but only 1 valence----Li • The number of valence electrons determines how many and what this atom (or ion) can bond to in order to make a mole ...
Atomic Structure and the Periodic Table
... 2. Work out which group the element is in and draw that number of electrons in the outer circle – with eight for Group 0 elements – except helium. 3. Fill the other circles with as many electrons as needed. Remember – two in the first circle, and eight in the second and third circles. 4. Finally, ch ...
... 2. Work out which group the element is in and draw that number of electrons in the outer circle – with eight for Group 0 elements – except helium. 3. Fill the other circles with as many electrons as needed. Remember – two in the first circle, and eight in the second and third circles. 4. Finally, ch ...
Word List
... 1.1 I can write the names and symbols of the elements in columns 1A – 4A on the periodic table. 1.5 I can write the names and symbols of the elements in columns 5A- 8A on the periodic table. 1.12 I can write the names and symbols of selected transition metals, lanthanides and actinides (1B12B) on th ...
... 1.1 I can write the names and symbols of the elements in columns 1A – 4A on the periodic table. 1.5 I can write the names and symbols of the elements in columns 5A- 8A on the periodic table. 1.12 I can write the names and symbols of selected transition metals, lanthanides and actinides (1B12B) on th ...
Isotopes and the Electron Configuration of the Blocks in the Periodic
... and the number of neutrons in it, the isotopes are determined by the condition A = Z + N, where A is the atomic mass, Z is the charge, N is the number of neutrons of the nucleus. With all these, it is necessary to keep in mind that, having the same number of protons in a nucleus, the nucleus may con ...
... and the number of neutrons in it, the isotopes are determined by the condition A = Z + N, where A is the atomic mass, Z is the charge, N is the number of neutrons of the nucleus. With all these, it is necessary to keep in mind that, having the same number of protons in a nucleus, the nucleus may con ...
Practice Test Chapters 17 & 18
... • Sr and Ca are in the same group so they are similar to each other • Sr has 18 extra electrons to cause harm • Sr and Ca are in the same period so are similar to each other • Sr is twice as massive as Calcium ...
... • Sr and Ca are in the same group so they are similar to each other • Sr has 18 extra electrons to cause harm • Sr and Ca are in the same period so are similar to each other • Sr is twice as massive as Calcium ...
atomic mass - Cloudfront.net
... because of its protons. • In a neutral atom the proton # = electron # • B = Boron = __p+, __e• Cl = Chlorine = ____p+, ____e- ...
... because of its protons. • In a neutral atom the proton # = electron # • B = Boron = __p+, __e• Cl = Chlorine = ____p+, ____e- ...
11129_evl_ch1_ste_corr
... d) Which of these elements are good conductors of electricity? Explain your answer. Sodium and magnesium are good conductors because they are both metals. ...
... d) Which of these elements are good conductors of electricity? Explain your answer. Sodium and magnesium are good conductors because they are both metals. ...
Atomic Structure
... • If there are either too many or not enough neutrons, a nucleus changes by giving out radiation. • This radiation may be in different forms • Already studied at IGCSE Physics! ...
... • If there are either too many or not enough neutrons, a nucleus changes by giving out radiation. • This radiation may be in different forms • Already studied at IGCSE Physics! ...
atomic number, mass, isotopes
... – use the element’s symbol and small dots to represent the valence electrons present in a specific atom ...
... – use the element’s symbol and small dots to represent the valence electrons present in a specific atom ...
atomic number
... You know that neutrons are found in the nucleus of an atom. Under normal conditions, protons and neutrons stick together in the nucleus. During radioactive decay, they may be knocked out of there. Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and ...
... You know that neutrons are found in the nucleus of an atom. Under normal conditions, protons and neutrons stick together in the nucleus. During radioactive decay, they may be knocked out of there. Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and ...
Atoms and Elements: Are they Related?
... • Which parts of an atom make up the mass of the atom? • Elements are made up of? • The element lead is made up of what kind of atoms? ...
... • Which parts of an atom make up the mass of the atom? • Elements are made up of? • The element lead is made up of what kind of atoms? ...
Introduction to Chemical Bonding
... The bond of Sodium and Fluorine is an example of Ionic bonding: electrons have been transferred in order for the atoms to have a full outer level. When an atom loses or gains electrons, it becomes what is called an ion. An ion is no longer neutrally charged because it has different numbers of proton ...
... The bond of Sodium and Fluorine is an example of Ionic bonding: electrons have been transferred in order for the atoms to have a full outer level. When an atom loses or gains electrons, it becomes what is called an ion. An ion is no longer neutrally charged because it has different numbers of proton ...
Bohr Models - Athena Chemistry
... 2. Compared to a proton, an electron has a) the same charge and mass b) the opposite charge and the same mass c) the opposite charge and less mass d) the opposite charge and greater mass 3. Which statement best describes the nucleus? a) it contains most of the mass of the atom and occupies the most ...
... 2. Compared to a proton, an electron has a) the same charge and mass b) the opposite charge and the same mass c) the opposite charge and less mass d) the opposite charge and greater mass 3. Which statement best describes the nucleus? a) it contains most of the mass of the atom and occupies the most ...
Isotopes - Katella HS
... Pennies vs Isotopes Both kinds have same identity Both have different masses because the insides are unique Unlike pennies atoms are too small to see ...
... Pennies vs Isotopes Both kinds have same identity Both have different masses because the insides are unique Unlike pennies atoms are too small to see ...
Isotopes - Katella HS
... Pennies vs Isotopes Both kinds have same identity Both have different masses because the insides are unique Unlike pennies atoms are too small to see ...
... Pennies vs Isotopes Both kinds have same identity Both have different masses because the insides are unique Unlike pennies atoms are too small to see ...
5.3 Representative Groups PPT
... n Relate the number of valence electrons to groups in the periodic table and to properties of elements in those groups. n Predict the reactivity of some elements based on their locations within a group. n Identify some properties of common A group elements. ...
... n Relate the number of valence electrons to groups in the periodic table and to properties of elements in those groups. n Predict the reactivity of some elements based on their locations within a group. n Identify some properties of common A group elements. ...
Atoms PowerPoint
... Occurs when atoms share electrons so that each atom can fill its valence shell some of the time Neither atom is strong enough to gain total control of any unpaired electrons There is an ongoing tug of war and these electrons remain attracted to both nuclei Occurs with atoms of 3, 4, 5, electrons in ...
... Occurs when atoms share electrons so that each atom can fill its valence shell some of the time Neither atom is strong enough to gain total control of any unpaired electrons There is an ongoing tug of war and these electrons remain attracted to both nuclei Occurs with atoms of 3, 4, 5, electrons in ...
Atoms and Electrons Name: Practice H
... 9. The light from fluorescent lights, when analyzed in a spectrometer, exhibit the same lines in the yellow, green and blue spectral regions. This is evidence that ...
... 9. The light from fluorescent lights, when analyzed in a spectrometer, exhibit the same lines in the yellow, green and blue spectral regions. This is evidence that ...