Chemistry - Target Publications
... (A) ∆H is positive, ∆S is positive (B) (C) ∆H is negative, ∆S is negative (D) ...
... (A) ∆H is positive, ∆S is positive (B) (C) ∆H is negative, ∆S is negative (D) ...
Acid K a
... How to write out Ka and Kb rxns and expressions. The weaker the acid the stronger the conjugate base (and vice versa). Conjugate bases of strong acids have no basic ...
... How to write out Ka and Kb rxns and expressions. The weaker the acid the stronger the conjugate base (and vice versa). Conjugate bases of strong acids have no basic ...
nomenclature review
... If 7.40g of calcium hydroxide reacts with excess nitric acid, how many grams of calcium nitrate are formed?(16.4grams) ...
... If 7.40g of calcium hydroxide reacts with excess nitric acid, how many grams of calcium nitrate are formed?(16.4grams) ...
enzyme
... (alkaline conditions), the COO- group will not be affected, but the -NH3+ group will lose a hydrogen ion. ...
... (alkaline conditions), the COO- group will not be affected, but the -NH3+ group will lose a hydrogen ion. ...
Chapter 17 Additional Aspects of Aqueous Equilibria I. Solubility
... Endpoint: the observed end of the titration (color change) Titration error: the difference between the observed and equivalence point. B. Strong base, weak acid acetic acid and sodium hydroxide leaves water and acetate ion which is basic. Therefore the titration ends with a basic pH greater than 7. ...
... Endpoint: the observed end of the titration (color change) Titration error: the difference between the observed and equivalence point. B. Strong base, weak acid acetic acid and sodium hydroxide leaves water and acetate ion which is basic. Therefore the titration ends with a basic pH greater than 7. ...
Chapter 14 - Hope Charter School
... b. best example is NaOH c. a good example is Ca(OH)2 d. based on the percentage of formula units that dissociate of those that dissolved e. Table 14.3 gives a list 2. Strong acids a. defined—an acid that completely ionizes in water b. best example is HCl c. a good example is H2SO4 d. based on the pe ...
... b. best example is NaOH c. a good example is Ca(OH)2 d. based on the percentage of formula units that dissociate of those that dissolved e. Table 14.3 gives a list 2. Strong acids a. defined—an acid that completely ionizes in water b. best example is HCl c. a good example is H2SO4 d. based on the pe ...
Honors Chemistry Chapter 14 notes—Acids, Bases, and pH I. Acids
... b. best example is NaOH c. a good example is Ca(OH)2 d. based on the percentage of formula units that dissociate of those that dissolved e. Table 14.3 gives a list 2. Strong acids a. defined—an acid that completely ionizes in water b. best example is HCl c. a good example is H2SO4 d. based on the pe ...
... b. best example is NaOH c. a good example is Ca(OH)2 d. based on the percentage of formula units that dissociate of those that dissolved e. Table 14.3 gives a list 2. Strong acids a. defined—an acid that completely ionizes in water b. best example is HCl c. a good example is H2SO4 d. based on the pe ...
The concept of pH and pKa
... • An acid (often represented by the generic formula HA [H+A-]) any chemical compound that, when dissolved in water, gives a solution with a hydrogen ion activity greater than in pure water (a pH less than 7.0) • an acid as a compound which donates a hydrogen ion (H+) to another compound (called a ...
... • An acid (often represented by the generic formula HA [H+A-]) any chemical compound that, when dissolved in water, gives a solution with a hydrogen ion activity greater than in pure water (a pH less than 7.0) • an acid as a compound which donates a hydrogen ion (H+) to another compound (called a ...
Chemical Bonding
... (10) Dissociation constants of carboxylic acids The dissociation constant of an acid depends on the stability of its ion. If the stability of anion of an acid is increased due to intramolecular hydrogen-bonding, the acid strength is greatly enhanced i.e., pKa value decreases. The carboxylate ion of ...
... (10) Dissociation constants of carboxylic acids The dissociation constant of an acid depends on the stability of its ion. If the stability of anion of an acid is increased due to intramolecular hydrogen-bonding, the acid strength is greatly enhanced i.e., pKa value decreases. The carboxylate ion of ...
Solution chemistry and reaction mechanism taking place during the
... In(OH)xSy contain InCl3, acetic acid and thioacetamide. As it was stated in a previous work [4], the chemical species present in the solution and the rate of thioacetamide hydrolysis, depend on the solution pH. Therefore, the pH of solutions containing thioacetamide, acetic acid and InCl3 was measur ...
... In(OH)xSy contain InCl3, acetic acid and thioacetamide. As it was stated in a previous work [4], the chemical species present in the solution and the rate of thioacetamide hydrolysis, depend on the solution pH. Therefore, the pH of solutions containing thioacetamide, acetic acid and InCl3 was measur ...
name - cloudfront.net
... 14.The Hall process for the production of aluminum involves the reaction of aluminum oxide with elemental carbon to give aluminum metal and carbon monoxide. If the yield of this reaction is 75%, what mass of aluminum metal can be produced from the reaction of 1.65 106 g of aluminum oxide with 1.50 ...
... 14.The Hall process for the production of aluminum involves the reaction of aluminum oxide with elemental carbon to give aluminum metal and carbon monoxide. If the yield of this reaction is 75%, what mass of aluminum metal can be produced from the reaction of 1.65 106 g of aluminum oxide with 1.50 ...
Storage Pattern for Chemicals Where Space is Limited
... schools try to use the excellent chemical storage system found in Flinn Scientific’s catalog. Unfortunately, many school stockrooms are too small to provide 23 separated locations for classes of chemicals. Here are some tips for creating safer chemical storage rooms: ...
... schools try to use the excellent chemical storage system found in Flinn Scientific’s catalog. Unfortunately, many school stockrooms are too small to provide 23 separated locations for classes of chemicals. Here are some tips for creating safer chemical storage rooms: ...
SAMPLE PAPER Class - XII SUBJECT
... But-2-enal Predict the products formed when cyclohexanecarbaldehyde reacts with Semicarbazide and weak acid 1 ...
... But-2-enal Predict the products formed when cyclohexanecarbaldehyde reacts with Semicarbazide and weak acid 1 ...
CHAPTER 8
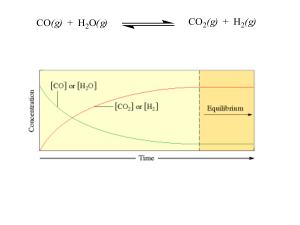
... reach equilibrium, reaction to stressors such as concentration, pressure or temperature changes, effect of catalysts. 6. Acids and bases: Definitions of Arrhenius, Bronsted-Lowry and Lewis acid/bases, conjugate acid/bases, strength of acid/base compared to water, equilibrium of water (Kw), pH, pOH, ...
... reach equilibrium, reaction to stressors such as concentration, pressure or temperature changes, effect of catalysts. 6. Acids and bases: Definitions of Arrhenius, Bronsted-Lowry and Lewis acid/bases, conjugate acid/bases, strength of acid/base compared to water, equilibrium of water (Kw), pH, pOH, ...
H 3 O + - St John Brebeuf
... Use a “⇄” and not “” It means they are Equilibrium situations! HF + H2O ⇄ H3O+ ...
... Use a “⇄” and not “” It means they are Equilibrium situations! HF + H2O ⇄ H3O+ ...