HSE Chemistry Questions
... NaCl ? ( mol. Wt. of NaCl=58.5 ) ( c ) For complete oxidation 60 ml of a ferrous sulphate solution with KMnO4 in acid medium the amount of 0.01 M K2Cr2O7 required for the same oxidation. ( d ) An aqueous solution is 0.01 M CH3OH. The concentration of the solution ...
... NaCl ? ( mol. Wt. of NaCl=58.5 ) ( c ) For complete oxidation 60 ml of a ferrous sulphate solution with KMnO4 in acid medium the amount of 0.01 M K2Cr2O7 required for the same oxidation. ( d ) An aqueous solution is 0.01 M CH3OH. The concentration of the solution ...
Ch2hon ppt part 3
... oxoacids, are as follows: 1. When all the H ions are removed from the “-ic” acid, the anion’s name ends with “-ate.” 2. When all the H ions are removed from the “ous” acid, the anion’s name ends with “-ite.” 3. The names of anions in which one or more but not all the hydrogen ions have been removed ...
... oxoacids, are as follows: 1. When all the H ions are removed from the “-ic” acid, the anion’s name ends with “-ate.” 2. When all the H ions are removed from the “ous” acid, the anion’s name ends with “-ite.” 3. The names of anions in which one or more but not all the hydrogen ions have been removed ...
analytical chemistry lecture 8
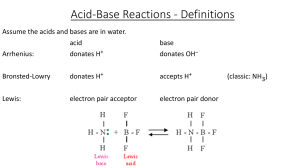
... ARRHENIUS THEORY-H+ AND OHˉ • An acid is any substance that ionizes (partially or completely) in water to give hydrogen ions • Means associate with solvent to give hydronium ions ...
... ARRHENIUS THEORY-H+ AND OHˉ • An acid is any substance that ionizes (partially or completely) in water to give hydrogen ions • Means associate with solvent to give hydronium ions ...
F324 summary - Macmillan Academy
... Hydrolysis and degradable polymers • Condensation polymers have chemical groups that are vulnerable to chemical attack from either acids or alkalis – polyesters (ester group) and polyamides (amide group). This process is known as hydrolysis and results in the breakdown of the polymer. • Disposing o ...
... Hydrolysis and degradable polymers • Condensation polymers have chemical groups that are vulnerable to chemical attack from either acids or alkalis – polyesters (ester group) and polyamides (amide group). This process is known as hydrolysis and results in the breakdown of the polymer. • Disposing o ...
Chemical Properties of Water - Part 2
... contains a steady state concentration of hydrogen ions and hydroxyl ions (both 10–7 M). ...
... contains a steady state concentration of hydrogen ions and hydroxyl ions (both 10–7 M). ...
SNC2D – Science 10 Tuesday April 26th, 2010 Mr. Sourlis and Mr
... b) Solid zinc metal reacts with aqueous hydrogen chloride to produce hydrogen gas (H2) and aqueous zinc chloride ...
... b) Solid zinc metal reacts with aqueous hydrogen chloride to produce hydrogen gas (H2) and aqueous zinc chloride ...
Chemical Equation Interpretations – Match the chemical equation
... carbonate in seashells and marble structures. This reaction produces calcium sulfate dissolved in water and carbon dioxide gas. ...
... carbonate in seashells and marble structures. This reaction produces calcium sulfate dissolved in water and carbon dioxide gas. ...
buffers - sbhschemistry
... 1. Calculate the pH of a 0.1molL-1 nitrous acid solution (HNO2) which has been combined with 0.1molL-1 sodium nitrite (NaNO2). Ka (HNO2) = 5 x 10-4 ...
... 1. Calculate the pH of a 0.1molL-1 nitrous acid solution (HNO2) which has been combined with 0.1molL-1 sodium nitrite (NaNO2). Ka (HNO2) = 5 x 10-4 ...
BITSAT Chemistry
... A mixture of C6H6 and excess H2 has a pressure of 60 mm of Hg in an unknown volume. After the gas had been passed over a nickel catalyst and all the benzene converted to cyclohexane, the pressure of the gas was 30 mm of Hg in the same volume at ...
... A mixture of C6H6 and excess H2 has a pressure of 60 mm of Hg in an unknown volume. After the gas had been passed over a nickel catalyst and all the benzene converted to cyclohexane, the pressure of the gas was 30 mm of Hg in the same volume at ...
Gen Chem Final--review problems Fall 2006
... charges on each atom, determine the molecular geometry, bond angles, whether or not the molecule is polar, and hybridization around the central atom. ...
... charges on each atom, determine the molecular geometry, bond angles, whether or not the molecule is polar, and hybridization around the central atom. ...
OVERVIEW DESCRIPTION DES ADVANTAGES LICENSING
... Lactic acid has been an intermediate-volume specialty chemical for a wide range of food processing and industrial applications that includes pharmaceutical, cosmetics and other chemicals synthesis. Researchers at the South Dakota School of Mines & Technology have developed a unique approach to synth ...
... Lactic acid has been an intermediate-volume specialty chemical for a wide range of food processing and industrial applications that includes pharmaceutical, cosmetics and other chemicals synthesis. Researchers at the South Dakota School of Mines & Technology have developed a unique approach to synth ...
Assistance Lecturer Amjad Ahmed Jumaa Arrhenius theory of acids
... If the correct amounts of acids and bases are mixed together, the original properties of the acid and bases are lost. The reaction product has a taste that is salty sour or bitter. A salt and water were formed when an acid neutralizes a base. For example: H2SO4 (aq) + 2KOH (aq) → Acid base ...
... If the correct amounts of acids and bases are mixed together, the original properties of the acid and bases are lost. The reaction product has a taste that is salty sour or bitter. A salt and water were formed when an acid neutralizes a base. For example: H2SO4 (aq) + 2KOH (aq) → Acid base ...
Acid-Base Reactions
... Acid-Base Titration What is the molar mass of H2X of 29.45 mL of 0.187 M NaOH are required to neutralize a solution prepared by adding 0.242 g of H2X to enough water to make 25.00 mL of solution? Plan: Determine number of moles of H2X Then you can calculate the mass, molar mass, and even volume or ...
... Acid-Base Titration What is the molar mass of H2X of 29.45 mL of 0.187 M NaOH are required to neutralize a solution prepared by adding 0.242 g of H2X to enough water to make 25.00 mL of solution? Plan: Determine number of moles of H2X Then you can calculate the mass, molar mass, and even volume or ...
Exam 2
... 6. In the reduction of 2-butanone to (2)-butanol using the (S)-CBS reagent (2-methyloxazaborolidine + BH3), what is transferred in the critical step in the reaction mechanism? a) a hydride ion, H- b) a hydrogen radical, H c) a proton, H+ d) both hydrogens simultaneously as molecular hydrogen, H2 e) ...
... 6. In the reduction of 2-butanone to (2)-butanol using the (S)-CBS reagent (2-methyloxazaborolidine + BH3), what is transferred in the critical step in the reaction mechanism? a) a hydride ion, H- b) a hydrogen radical, H c) a proton, H+ d) both hydrogens simultaneously as molecular hydrogen, H2 e) ...