* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organic Chemistry Fifth Edition
Survey
Document related concepts
Biological aspects of fluorine wikipedia , lookup
Citric acid cycle wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Peptide synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Genetic code wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Transcript
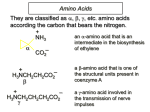
27.1 Classification of Amino Acids Classification of Amino Acids amino acids are classified as , , , etc. to indicate where the nitrogen atom is relative to the carboxylic acid: O H H O N H N H O H O N -Amino Acid -Amino Acid H O N H H -Amino Acid O Selected Amino Acids 1-Aminocyclopropanecarboxylic Acid NH2 an -amino acid that is an intermediate in the biosynthesis of ethylene OH O H 3-Aminopropanoic Acid O H N O N H N H O H O a -amino acid that is one of the structural units present in coenzyme A 4-Aminobutanoic Acid a -amino acid involved in the transmission of nerve impulses -Amino Acids are Most Ubiquitous in Nature H H O N N O H O N -Amino Acid -Amino Acid H O N H H H O H -Amino Acid O Revision: Amines are Brønsted Bases N H X N X Amines are electron-rich and have a reactive lone pair, which can form a covalent bond to a proton to form an ammonium ion. Amino Acids Exist as Zwitterions While their name implies that amino acids are compounds that contain an amine (— NH2) and a carboxylic acid (-CO2H), these groups are actually present as their conjugate acid (—NH3+) and conjugate base (—CO2–), respectively. Amino Acids Exist as Zwitterions Basic Amine H H O N H H H H O H O N Acidic Carboxylic Acid H O H N Resonance-Stabilized Zwitterion O O Zwitterions Defined Zwitterionic Compounds/Zwitterions neutral compounds having formal unit electrical charges of opposite sign. Some chemists restrict the term to compounds with the charges on nonadjacent atoms. Sometimes referred to as inner salts, dipolar ions (a misnomer), e.g. +H3N-CH2CO2ammonioacetate (glycine). IUPAC Compendium of Chemical Terminology Amino Acids Exist as Zwitterions H H O H H N N H O O H O What evidence do we have for this behavior……… Properties of Glycine Reflect its Zwitterionic Structure High Water Solubility glycine is soluble in water but not in nonpolar solvents. High Melting Point when heated to 233°C it decomposes before it melts. Properties of Glycine Reflect its Zwitterionic Structure The physical properties of glycine are consistent with this structure H O H O H N H H O N H O Polar Zwitterion 27.2 Stereochemistry of -Amino Acids Non-Polar Side Chains: Alanine O Alanine (Ala, A) H3N Me CO2 O H H3N H Me Alanine, valine, leucine, and isoleucine have alkyl groups as side chains, which are non-polar and hydrophobic Non-Polar Side Chains: Valine O Valine (Val, V) H3N Me CO2 O H3N H H Me Me Me Non-Polar Side Chains: Phenylalanine O Phenylalanine (Phe, F) H3N CO2 O H3N H The side chain in phenylalanine (a nonpolar amino acid) is a benzyl group. H