Lokshin2011
... from tricarbonyl complexes and dicarbonyl chelates are formed, stabilized by intramolecular coordination of the manganese atom with a substituent in the Cp-ring. This changes the color of the solution. In a closed system the CO molecules released during irradiation adds again to the intermediate and ...
... from tricarbonyl complexes and dicarbonyl chelates are formed, stabilized by intramolecular coordination of the manganese atom with a substituent in the Cp-ring. This changes the color of the solution. In a closed system the CO molecules released during irradiation adds again to the intermediate and ...
6 Chemical Bonding – Orbital Theory
... formed after mixing, is invariably equal to the number of atomic orbitals mixed or hybridized. An important characteristic of hybrid orbitals is that they are all identical in respect of energy and directional character. They, however, differ from the original atomic orbitals in these respects. They ...
... formed after mixing, is invariably equal to the number of atomic orbitals mixed or hybridized. An important characteristic of hybrid orbitals is that they are all identical in respect of energy and directional character. They, however, differ from the original atomic orbitals in these respects. They ...
Organic Chemistry II / CHEM 252 Chapter 16
... • Dissolving aldehydes (or ketones) in water causes formation of an equilibrium between the carbonyl compound and its hydrate – The hydrate is also called a gem-diol (gem i.e. geminal, indicates the presence of two identical substituents on the same carbon) – The equilibrum favors a ketone over its ...
... • Dissolving aldehydes (or ketones) in water causes formation of an equilibrium between the carbonyl compound and its hydrate – The hydrate is also called a gem-diol (gem i.e. geminal, indicates the presence of two identical substituents on the same carbon) – The equilibrum favors a ketone over its ...
Chapter 18: Organic Chemistry
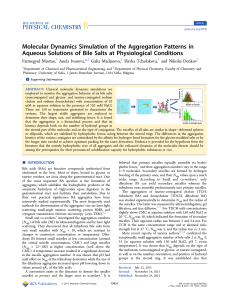
... 1) Find the longest carbon chain containing the double bond - this is the parent chain. Name the parent chain like an alkane, but drop the 'ane' ending and add 'ene'. 2) Number the parent chain starting from the end closer to the double bond and designate the location of the first carbon in the doub ...
... 1) Find the longest carbon chain containing the double bond - this is the parent chain. Name the parent chain like an alkane, but drop the 'ane' ending and add 'ene'. 2) Number the parent chain starting from the end closer to the double bond and designate the location of the first carbon in the doub ...
c8h18 isomers
... ♦ The molecules are non-polar or very weakly polar ♦ The forces holding together non-polar molecules are van der Waals forces. These intermolecular forces, which operate only over very small distances, result from induced polarization of the electron clouds in molecules. ♦ Within a family: The large ...
... ♦ The molecules are non-polar or very weakly polar ♦ The forces holding together non-polar molecules are van der Waals forces. These intermolecular forces, which operate only over very small distances, result from induced polarization of the electron clouds in molecules. ♦ Within a family: The large ...
Learning Guide for Chapter 5 - NMR Spectroscopy
... Learning Guide for Chapter 5 - NMR Spectroscopy I. Introduction to NMR spectroscopy - p 1 II. Distinguishing equivalent H's - p 3 III. Chemical shift - p 4 IV. Integration - p 7 V. Spin-spin Splitting - p 9 VI. Practice with NMR spectra VI. Deuterium in NMR - p 12 VII. Carbon-13 NMR- p 13 I. Introdu ...
... Learning Guide for Chapter 5 - NMR Spectroscopy I. Introduction to NMR spectroscopy - p 1 II. Distinguishing equivalent H's - p 3 III. Chemical shift - p 4 IV. Integration - p 7 V. Spin-spin Splitting - p 9 VI. Practice with NMR spectra VI. Deuterium in NMR - p 12 VII. Carbon-13 NMR- p 13 I. Introdu ...
CHAPTER 15
... (1) In the IUPAC nomenclature system, an aldehyde group has priority over a ketone group. (2) Addition of an alcohol molecule across the carbon-oxygen double bond of an aldehyde produces a compound in which the carbonyl carbon atom bears both an alkoxy group and a hydroxy group. (3) 2-Propenal conta ...
... (1) In the IUPAC nomenclature system, an aldehyde group has priority over a ketone group. (2) Addition of an alcohol molecule across the carbon-oxygen double bond of an aldehyde produces a compound in which the carbonyl carbon atom bears both an alkoxy group and a hydroxy group. (3) 2-Propenal conta ...
Biomimetic Organic Synthesis. 2 Volume Set Brochure
... Biomimetic Organic Synthesis. 2 Volume Set Description: ...
... Biomimetic Organic Synthesis. 2 Volume Set Description: ...
Reductive Coupling Reactions of Nitrones and Imines
... explained by the preferred orientation shown in Scheme 3.7 The ortho substituent introduces chirality into the ferrocene, which adopts the thermodynamically more stable anti conformation of the C=N bond. This conformational stability inhibits bond rotation about the ferrocene-C=N bond, providing the ...
... explained by the preferred orientation shown in Scheme 3.7 The ortho substituent introduces chirality into the ferrocene, which adopts the thermodynamically more stable anti conformation of the C=N bond. This conformational stability inhibits bond rotation about the ferrocene-C=N bond, providing the ...
Drawing Organic Structures Functional Groups Constitutional Isomers
... tertiary amines are all stronger bases than ammonia Note pKa values for conjugate acid Lower pKa indicates weaker base ...
... tertiary amines are all stronger bases than ammonia Note pKa values for conjugate acid Lower pKa indicates weaker base ...
Alcohols - Chem1-tsu
... the sp3 hybridized oxygen are arranged approximately in a tetrahedral arrangement. This bond angle is slightly greater than the tetrahedral angle due to the repulsive interaction between the two bulky (-R) groups. The C - O bond length (141 pm) in ethers is almost as same as in alcohols. ...
... the sp3 hybridized oxygen are arranged approximately in a tetrahedral arrangement. This bond angle is slightly greater than the tetrahedral angle due to the repulsive interaction between the two bulky (-R) groups. The C - O bond length (141 pm) in ethers is almost as same as in alcohols. ...
Advanced Practical Organic Chemistry
... expression CnH2n+2. The structural formula, shown for the first five alkanes in the table, shows each carbon atom and the elements that are attached to it. This structural formula is important when we begin to discuss more complex hydrocarbons. The simple alkanes share many properties in common. All ...
... expression CnH2n+2. The structural formula, shown for the first five alkanes in the table, shows each carbon atom and the elements that are attached to it. This structural formula is important when we begin to discuss more complex hydrocarbons. The simple alkanes share many properties in common. All ...
Alkenes notes
... In double bonds, the first bond involves an overlap of atomic orbitals directly in between the nuclei of the two atoms: ...
... In double bonds, the first bond involves an overlap of atomic orbitals directly in between the nuclei of the two atoms: ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.