Organic Chemistry II
... Alkyl Halides We did talk about halo-alkanes (called alkyl halides) which are alkanes with a halogen attached. These molecules do, in fact, have polar bonds: C-Br, C-I, C-Cl are all polar bonds. Carbon is slightly positive, the halogen is slightly negative. ...
... Alkyl Halides We did talk about halo-alkanes (called alkyl halides) which are alkanes with a halogen attached. These molecules do, in fact, have polar bonds: C-Br, C-I, C-Cl are all polar bonds. Carbon is slightly positive, the halogen is slightly negative. ...
Organic Halides (Haloalkanes) (Alkyl Halides)
... (their use is now restricted due to the fact that some of them damage the ozone layer) • polymers such as Teflon (non-stick surfaces as in cookware) and PVC (polyvinylchloride) used plumbing • DDT (dichlorodiphenyltrichloroethane) – now banned pesticide • PCB’s (polychlorinated biphenyls) – electric ...
... (their use is now restricted due to the fact that some of them damage the ozone layer) • polymers such as Teflon (non-stick surfaces as in cookware) and PVC (polyvinylchloride) used plumbing • DDT (dichlorodiphenyltrichloroethane) – now banned pesticide • PCB’s (polychlorinated biphenyls) – electric ...
Chapter 4
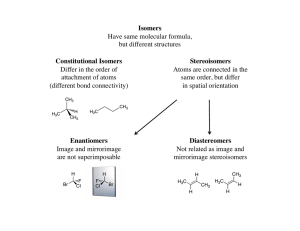
... Therefore if a given solution has 90% of one enantiomer (say R) and 10% of the other enantiomer (S) then the enantiomeric excess is 80% ...
... Therefore if a given solution has 90% of one enantiomer (say R) and 10% of the other enantiomer (S) then the enantiomeric excess is 80% ...
DEPARTMENT OF CHEMISTRY, UNIVERSITY OF JYVÄSKYLÄ
... chemical bonds has been a priority for chemists for hundreds of years. The modern description of chemical bonding is based on quantum theory and the atomic shell structure, which easily rationalize why bonding is typically far simpler for s- and p-block elements than for their d- and f-block counter ...
... chemical bonds has been a priority for chemists for hundreds of years. The modern description of chemical bonding is based on quantum theory and the atomic shell structure, which easily rationalize why bonding is typically far simpler for s- and p-block elements than for their d- and f-block counter ...
Ch-6-Alcohols and phenols - Home
... tissue that it comes into contact with. This gives severe skin burning and if inhaled serious internal corrosion. The skin burning is not initially felt because the phenol has a local anaesthetic effect. It can affect the central nervous system, which will at first lead to sweating, weakness, dizzin ...
... tissue that it comes into contact with. This gives severe skin burning and if inhaled serious internal corrosion. The skin burning is not initially felt because the phenol has a local anaesthetic effect. It can affect the central nervous system, which will at first lead to sweating, weakness, dizzin ...
CH 3 - bYTEBoss
... • Organic molecules exhibit three different types of hybridization at the carbon center: – sp3 hybridized carbons for tetrahedral geometries; – sp2 hybridized carbons for trigonal planar geometries; and – sp hybridized carbons for linear geometries. ...
... • Organic molecules exhibit three different types of hybridization at the carbon center: – sp3 hybridized carbons for tetrahedral geometries; – sp2 hybridized carbons for trigonal planar geometries; and – sp hybridized carbons for linear geometries. ...
Chemistry 30 – Organic Chemistry
... compounds from living or once-living organisms • Wohler, 1828, synthesized urea (an organic compound) from inorganic chemicals • Today organic compounds defined to be molecular compounds of carbon – exception: oxides of carbon – CO, CO2 ...
... compounds from living or once-living organisms • Wohler, 1828, synthesized urea (an organic compound) from inorganic chemicals • Today organic compounds defined to be molecular compounds of carbon – exception: oxides of carbon – CO, CO2 ...
Document
... Silicone rubber offers good resistance to extreme temperatures,being able to operate normally from -55°C to +300°C.At the extreme temperatures, the tensile strength, elongation, tear strength and compression set can be far superior to conventional rubbers although still low relative to other materia ...
... Silicone rubber offers good resistance to extreme temperatures,being able to operate normally from -55°C to +300°C.At the extreme temperatures, the tensile strength, elongation, tear strength and compression set can be far superior to conventional rubbers although still low relative to other materia ...
File - Chemistry Workshop
... A tertiary carbon is directly bonded to three other C’s. Multivalent atoms are 1º, 2º, or 3º by bonding to C’s. Univalent atom or group not really 1º, 2º, or 3º on its own - ID depends on type of carbon it is bonded to. ...
... A tertiary carbon is directly bonded to three other C’s. Multivalent atoms are 1º, 2º, or 3º by bonding to C’s. Univalent atom or group not really 1º, 2º, or 3º on its own - ID depends on type of carbon it is bonded to. ...
phenol
... double bond and is referred to as hydroxylation. Both oxygens of the diol come from osmium tetraoxide via the cyclic osmate ester. The reaction of OsO4 with the alkene is a syn addition, and the conversion of the cyclic osmate to the diol involves cleavage of the bonds between oxygen and osmium. Thu ...
... double bond and is referred to as hydroxylation. Both oxygens of the diol come from osmium tetraoxide via the cyclic osmate ester. The reaction of OsO4 with the alkene is a syn addition, and the conversion of the cyclic osmate to the diol involves cleavage of the bonds between oxygen and osmium. Thu ...
Organic for Forensic Science
... Functional Groups Functional groups are • a characteristic feature of organic molecules that behave in a predictable, similar way. • composed of an atom or group of atoms. • groups that replace a hydrogen atom in the corresponding alkane. • a way to classify families of organic compounds. ...
... Functional Groups Functional groups are • a characteristic feature of organic molecules that behave in a predictable, similar way. • composed of an atom or group of atoms. • groups that replace a hydrogen atom in the corresponding alkane. • a way to classify families of organic compounds. ...
Catalytic hydrogenation
... The catalytic hydrogenation of polar C=O and C=N bonds are key reactions in fine chemical and pharmaceutical synthesis. A very important group of catalysts operate by hydride transfer to the substrate in the outer coordination sphere of the complex. Hydrogen can come from H2 or from an organic donor ...
... The catalytic hydrogenation of polar C=O and C=N bonds are key reactions in fine chemical and pharmaceutical synthesis. A very important group of catalysts operate by hydride transfer to the substrate in the outer coordination sphere of the complex. Hydrogen can come from H2 or from an organic donor ...
COURSES SCHEME & SYLLABUS
... Photo-Fries reactions of Anilides. Photo-Fries rearrangement. Barton reaction. Aromaticity: Aromaticity in benzenoid and non-benzenoid compounds, Alternant and nonalternant hydrocarbons, Huckel's rule, Energy level of -molecular orbitals, Annulenes, Antiaromaticity, Homo-aromaticity. Course Learnin ...
... Photo-Fries reactions of Anilides. Photo-Fries rearrangement. Barton reaction. Aromaticity: Aromaticity in benzenoid and non-benzenoid compounds, Alternant and nonalternant hydrocarbons, Huckel's rule, Energy level of -molecular orbitals, Annulenes, Antiaromaticity, Homo-aromaticity. Course Learnin ...
Nomenclature Chapter
... R = any general carbon group (it sometimes includes hydrogen too) Ar = any general aromatic group, (when more specificity than ‘just’ R is desired) The foundation of organic nomenclature requires an ability to name alkanes, alkenes and alkynes. Learning the rules for these groups will be your bigges ...
... R = any general carbon group (it sometimes includes hydrogen too) Ar = any general aromatic group, (when more specificity than ‘just’ R is desired) The foundation of organic nomenclature requires an ability to name alkanes, alkenes and alkynes. Learning the rules for these groups will be your bigges ...
BIORANSFORMATION
... • Most drugs are excreted by the kidneys. • For renal excretion drugs should: – have small molecular mass – be polar in nature – not be fully ionised at body pH • Most drugs are complex and do not have these properties and thus have to be broken down to simpler products. • Drugs are lipophilic in na ...
... • Most drugs are excreted by the kidneys. • For renal excretion drugs should: – have small molecular mass – be polar in nature – not be fully ionised at body pH • Most drugs are complex and do not have these properties and thus have to be broken down to simpler products. • Drugs are lipophilic in na ...
Alkenes 3 - ChemWeb (UCC)
... This reaction illustrated above is called a 1,2- or -elimination to indicate that the groups being eliminated are located on adjacent atoms in the starting material as compared to a 1,1- or -elimination where both are located on the same carbon atom This, in itself, tells you nothing about the act ...
... This reaction illustrated above is called a 1,2- or -elimination to indicate that the groups being eliminated are located on adjacent atoms in the starting material as compared to a 1,1- or -elimination where both are located on the same carbon atom This, in itself, tells you nothing about the act ...
organic practice problems
... 1. Carbon shows a very strong tendency to form a. ionic bonds. c. hydrogen bonds. b. covalent bonds. d. highly polar bonds. 2. How many outermost electrons does a carbon atom have? a. 3 c. 5 b. 4 d. 6 3. How many single covalent bonds can a carbon atom form? a. 2 c. 4 b. 3 d. 5 4. When a carbon atom ...
... 1. Carbon shows a very strong tendency to form a. ionic bonds. c. hydrogen bonds. b. covalent bonds. d. highly polar bonds. 2. How many outermost electrons does a carbon atom have? a. 3 c. 5 b. 4 d. 6 3. How many single covalent bonds can a carbon atom form? a. 2 c. 4 b. 3 d. 5 4. When a carbon atom ...
Full file at http://testbanksolution.eu/Test-Bank-Bank-for
... d. All resonance structures must have the same number of electrons ANS: A 50. Which of the following statements is not true about the carbonate anion, CO32? a. All of the oxygen atoms bear the same amount of charge b. All of the carbon-oxygen bonds are the same length c. The carbon atom bears the n ...
... d. All resonance structures must have the same number of electrons ANS: A 50. Which of the following statements is not true about the carbonate anion, CO32? a. All of the oxygen atoms bear the same amount of charge b. All of the carbon-oxygen bonds are the same length c. The carbon atom bears the n ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.