MAJOR - Bijni College
... Plots of and ψ2 for 1s,2s,2p,2px,2py,2pz,3dxy,3dzx orbital. n,l,m quantum numbersorigin and significance(outline only). i) The concept of spin and spin quantum numbers (outline only).Many electron atoms. Electron repulsion in the He atom. Pauli’s exclusion principle. Aufbau principle and electron co ...
... Plots of and ψ2 for 1s,2s,2p,2px,2py,2pz,3dxy,3dzx orbital. n,l,m quantum numbersorigin and significance(outline only). i) The concept of spin and spin quantum numbers (outline only).Many electron atoms. Electron repulsion in the He atom. Pauli’s exclusion principle. Aufbau principle and electron co ...
Conjugate addition_Clayden
... The reason that α,β-unsaturated carbonyl compounds react differently is conjugation, the phenomenon we discussed in Chapter 7. There we introduced you to the idea that bringing two π systems (two C=C bonds, for example, or a C=C bond and a C=O bond) close together leads to a stabilizing interaction. ...
... The reason that α,β-unsaturated carbonyl compounds react differently is conjugation, the phenomenon we discussed in Chapter 7. There we introduced you to the idea that bringing two π systems (two C=C bonds, for example, or a C=C bond and a C=O bond) close together leads to a stabilizing interaction. ...
Unit 13: Organic Chemistry
... O2 (diatomic oxygen), and O3 (ozone). 9. Amide: A hydrocarbon with a –CO-NH- (amide) group substituted onto the primary hydrocarbon chain. 10. Amine: A hydrocarbon with a –N= (amine) group substituted onto the primary hydrocarbon chain. 11. Combustion: A form of reaction where a hydrocarbon reacts w ...
... O2 (diatomic oxygen), and O3 (ozone). 9. Amide: A hydrocarbon with a –CO-NH- (amide) group substituted onto the primary hydrocarbon chain. 10. Amine: A hydrocarbon with a –N= (amine) group substituted onto the primary hydrocarbon chain. 11. Combustion: A form of reaction where a hydrocarbon reacts w ...
10.1 Intro to Organic Chemistry 10.1 Organic Chemistry
... 10.1.7 Deduce structural formulas for the isomers of the straightchain alkenes up to C6. 10.1.8 Apply IUPAC rules for naming the isomers of the straight-chain alkenes up to C6. 10.1.9 Deduce structural formulas for compounds containing up to six carbon atoms with one of the following functional grou ...
... 10.1.7 Deduce structural formulas for the isomers of the straightchain alkenes up to C6. 10.1.8 Apply IUPAC rules for naming the isomers of the straight-chain alkenes up to C6. 10.1.9 Deduce structural formulas for compounds containing up to six carbon atoms with one of the following functional grou ...
Unit 13: Organic Chemistry
... Objective: What are Alkenes, and how do they function in chemistry? Alkene Family: 1. The alkene family, also known as the olefin family, differ from their related alkanes by having one carbon to carbon double bond (C=C) somewhere along the longest chain. 2. Ethane (C2H4) and propene (C3H6) are the ...
... Objective: What are Alkenes, and how do they function in chemistry? Alkene Family: 1. The alkene family, also known as the olefin family, differ from their related alkanes by having one carbon to carbon double bond (C=C) somewhere along the longest chain. 2. Ethane (C2H4) and propene (C3H6) are the ...
Functional Groups
... Organic Molecules and Functional Groups Functional Groups: • Ethane: This molecule has only C—C and C—H bonds, so it has no functional group. It has no polar bonds, no lone pairs, and so it has no reactive sites. It is very unreactive. • Ethanol: This molecule has an OH group attached to its backbo ...
... Organic Molecules and Functional Groups Functional Groups: • Ethane: This molecule has only C—C and C—H bonds, so it has no functional group. It has no polar bonds, no lone pairs, and so it has no reactive sites. It is very unreactive. • Ethanol: This molecule has an OH group attached to its backbo ...
Chapter 1 Review Questions
... positively or negatively charged areas. In this case, water molecules are not attracted to these substances. Instead, water molecules attract each other. Since the oil and the water remain separated (do not mix), water is, therefore, a poor solvent for oil. 27. Given the large number of manufacture ...
... positively or negatively charged areas. In this case, water molecules are not attracted to these substances. Instead, water molecules attract each other. Since the oil and the water remain separated (do not mix), water is, therefore, a poor solvent for oil. 27. Given the large number of manufacture ...
Highly Enantioselective Cyclocarbonylation of Allylic
... of the palladium hydride to the allylic CdC bond as proposed by Alper. A notable advance emerging from these studies is the asymmetric cyclocarbonylation of the six-membered ring allylicalcohol 3e using a Pd-BICP catalyst. At 80 °C, chiral γ-butyrolactone 4e was formed in 93% ee and 87% yield (entry ...
... of the palladium hydride to the allylic CdC bond as proposed by Alper. A notable advance emerging from these studies is the asymmetric cyclocarbonylation of the six-membered ring allylicalcohol 3e using a Pd-BICP catalyst. At 80 °C, chiral γ-butyrolactone 4e was formed in 93% ee and 87% yield (entry ...
Alcohols and Phenols
... not), forming soluble salts that are soluble in dilute aqueous • A phenolic component can be separated from an organic solution by extraction into basic aqueous solution and is isolated after acid is added to the solution ...
... not), forming soluble salts that are soluble in dilute aqueous • A phenolic component can be separated from an organic solution by extraction into basic aqueous solution and is isolated after acid is added to the solution ...
Properties of Ionic Compounds
... For sodium chloride, the lowest wholenumber ratio of the ions is 1:1 (one Na+ ion to each Cℓ– ion). • The formula unit for sodium chloride is NaCℓ. • Although ionic charges are used to derive the correct formula, they are not shown when you write the formula unit of the compound. ...
... For sodium chloride, the lowest wholenumber ratio of the ions is 1:1 (one Na+ ion to each Cℓ– ion). • The formula unit for sodium chloride is NaCℓ. • Although ionic charges are used to derive the correct formula, they are not shown when you write the formula unit of the compound. ...
Ir-catalysed formation of C− F bonds. From allylic alcohols to α
... ketones as single constitutional isomers is reported. The introduction of fluorine into organic compounds has become a widely applied strategy to modulate the steric, electronic and lipophilic properties of a molecule. In this way, fluorine atoms can be used to modify the pharmacokinetic properties of ...
... ketones as single constitutional isomers is reported. The introduction of fluorine into organic compounds has become a widely applied strategy to modulate the steric, electronic and lipophilic properties of a molecule. In this way, fluorine atoms can be used to modify the pharmacokinetic properties of ...
In the data set I send you there are the compounds with some
... ID: the number as in EPA list. The original data set of EPA is bigger. For instance it contains compounds without toxicity values. I have cut these compounds, but left the original ID number in the EPA list. NAME: the chemical name CAS: the CAS number The following five columns (in red) are fr ...
... ID: the number as in EPA list. The original data set of EPA is bigger. For instance it contains compounds without toxicity values. I have cut these compounds, but left the original ID number in the EPA list. NAME: the chemical name CAS: the CAS number The following five columns (in red) are fr ...
SYNTHESIS OF NEW DICLOFENAC DERIVATIVES BY COUPLING WITH CHALCONE
... Methods: Mutual prodrugs were synthesized by conjugation of Diclofenac with a series of eight chalcone derivatives by the Claisen–Schmidt condensation of acetophenone or p-hydroxyacetophenone with benzaldehyde or appropriately substituted benzaldehyde in the presence of SOCl2/EtOH as a catalyst. Res ...
... Methods: Mutual prodrugs were synthesized by conjugation of Diclofenac with a series of eight chalcone derivatives by the Claisen–Schmidt condensation of acetophenone or p-hydroxyacetophenone with benzaldehyde or appropriately substituted benzaldehyde in the presence of SOCl2/EtOH as a catalyst. Res ...
State the main methods used to prepare polymers?
... c) This is in line with Markovnikov's Rule which says: When a compound HX is added to an unsymmetrical alkene, the hydrogen becomes attached to the carbon with the most hydrogens attached to it already. In this case, the hydrogen becomes attached to the CH2 group, because the CH2 group has more hydr ...
... c) This is in line with Markovnikov's Rule which says: When a compound HX is added to an unsymmetrical alkene, the hydrogen becomes attached to the carbon with the most hydrogens attached to it already. In this case, the hydrogen becomes attached to the CH2 group, because the CH2 group has more hydr ...
Amines
... are used to indicate that nitrogen has replaced carbon in the corresponding hydrocarbon H The nitrogen is assigned position 1 and the ring is numbered to give the lowest overall set of locants to the heteroatoms ...
... are used to indicate that nitrogen has replaced carbon in the corresponding hydrocarbon H The nitrogen is assigned position 1 and the ring is numbered to give the lowest overall set of locants to the heteroatoms ...
幻灯片 1
... If a double or triple bond is present, the parent chain must contain it. • Rule 2 Number the carbon atoms of the parent chain, beginning at the end nearer the first substituent, regardless of whether it is alkyl or halo. Assign each substituent a number to its position on the chain. • Rule 3 If the ...
... If a double or triple bond is present, the parent chain must contain it. • Rule 2 Number the carbon atoms of the parent chain, beginning at the end nearer the first substituent, regardless of whether it is alkyl or halo. Assign each substituent a number to its position on the chain. • Rule 3 If the ...
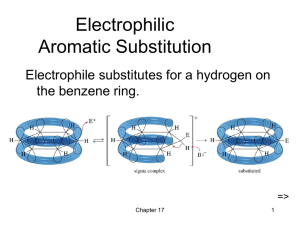
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.