Ethers and Epoxides
... These 3 membered rings are named using the term “epoxy” as one substituent bridging two adjacent C atoms. (NOT like cyclopropane) ...
... These 3 membered rings are named using the term “epoxy” as one substituent bridging two adjacent C atoms. (NOT like cyclopropane) ...
bonding
... Alkenes Stereoisomers - recall cycloalkane stereoisomers: substituents are either on the same side of the ring (cis) or on opposite sides (trans). ...
... Alkenes Stereoisomers - recall cycloalkane stereoisomers: substituents are either on the same side of the ring (cis) or on opposite sides (trans). ...
Ch14 Lecture
... • The hair is then turned around curlers, and an oxidizing agent is applied. • This re-forms the disulfide bonds in the hair, now giving it a curly appearance. ...
... • The hair is then turned around curlers, and an oxidizing agent is applied. • This re-forms the disulfide bonds in the hair, now giving it a curly appearance. ...
Infrared Radiation Infrared radiation (IR) is the term we use to
... group is found. Consequently, tables of group frequencies are available and if a compound shows absorptions characteristic of a particular group it's a good guess that that group is present. One such table can be found at http://www.cem.msu.edu/~reusch/VirtualText/Spectrpy/InfraRed/infrared.htm ...
... group is found. Consequently, tables of group frequencies are available and if a compound shows absorptions characteristic of a particular group it's a good guess that that group is present. One such table can be found at http://www.cem.msu.edu/~reusch/VirtualText/Spectrpy/InfraRed/infrared.htm ...
Chapter 10
... The reason is due to a type of resonance with the neighboring C-H bond called “hyperconjugation” ...
... The reason is due to a type of resonance with the neighboring C-H bond called “hyperconjugation” ...
Teaching with SCIGRESS - Photochemical Dynamics Group
... successfully predicts molecular geometry, neither theory takes into account the relationship between atomic orbitals and electrons in bonds. Valence-bond theory approaches this by describing bonding as the overlap of atomic orbitals from two bonded atoms and explains molecular geometry using hybrid ...
... successfully predicts molecular geometry, neither theory takes into account the relationship between atomic orbitals and electrons in bonds. Valence-bond theory approaches this by describing bonding as the overlap of atomic orbitals from two bonded atoms and explains molecular geometry using hybrid ...
Organic and Biochemical Molecules
... Geometric isomers of alkenes – If each sp2 C has 2 different groups attached it will have two possible orientations due the fact that pi bonds do not allow for free rotation – If each sp2 C has only one hydrogen you can classify the structure as cis or trans ...
... Geometric isomers of alkenes – If each sp2 C has 2 different groups attached it will have two possible orientations due the fact that pi bonds do not allow for free rotation – If each sp2 C has only one hydrogen you can classify the structure as cis or trans ...
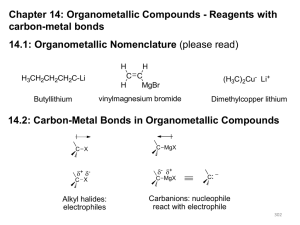
Organometallic Compounds - Reagents
... to a starting compound using known and reliable reactions. “it is a problem solving technique for transforming the structure of a synthetic target molecule (TM) to a sequence of progressively simpler structures along the pathway which ultimately leads to simple or commercially available starting mat ...
... to a starting compound using known and reliable reactions. “it is a problem solving technique for transforming the structure of a synthetic target molecule (TM) to a sequence of progressively simpler structures along the pathway which ultimately leads to simple or commercially available starting mat ...
Organometallic Chemistry
... Utilization of Organomagnesium reagents • In spite of versatility and broad synthetic utility of the Grignard reaction for C-C bond formation, it is often accompanied by competing side reactions such as enolization, reduction, or aldol condensation of the carbonyl substrate. • Organomagnesium compo ...
... Utilization of Organomagnesium reagents • In spite of versatility and broad synthetic utility of the Grignard reaction for C-C bond formation, it is often accompanied by competing side reactions such as enolization, reduction, or aldol condensation of the carbonyl substrate. • Organomagnesium compo ...
Grignard Reagents
... to a starting compound using known and reliable reactions. “it is a problem solving technique for transforming the structure of a synthetic target molecule (TM) to a sequence of progressively simpler structures along the pathway which ultimately leads to simple or commercially available starting mat ...
... to a starting compound using known and reliable reactions. “it is a problem solving technique for transforming the structure of a synthetic target molecule (TM) to a sequence of progressively simpler structures along the pathway which ultimately leads to simple or commercially available starting mat ...
Amine‐Directed Hydrogen‐Bonded Two‐Dimensional
... first choice in manufacturing sensors,[10] electronics[9] or switches at the single-molecule level.[5, 11–14] One possible strategy can be employing pH, due to applications in designing experiments in aqueous solutions under potential control,[15–18] as a knob to manipulate intermolecular hydrogen b ...
... first choice in manufacturing sensors,[10] electronics[9] or switches at the single-molecule level.[5, 11–14] One possible strategy can be employing pH, due to applications in designing experiments in aqueous solutions under potential control,[15–18] as a knob to manipulate intermolecular hydrogen b ...
Organic Stereochemistry. Part 2
... Fig. 2.11. Aromatic rings deserve a special explanation. They are treated as Kekulé structures (i.e., with alternating single and double bonds). For aryl groups such as phenyl (2.49) or naphthyl, it does not matter which resonance form is used, because splitting the double bonds gives the same resu ...
... Fig. 2.11. Aromatic rings deserve a special explanation. They are treated as Kekulé structures (i.e., with alternating single and double bonds). For aryl groups such as phenyl (2.49) or naphthyl, it does not matter which resonance form is used, because splitting the double bonds gives the same resu ...
Lecture - Ch 24
... • Electrophilic aromatic substitution – Amino substituents are strongly activating and ortho- and para-directing groups in electrophilic aromatic substitution reactions – Reactions are controlled by conversion to amide ...
... • Electrophilic aromatic substitution – Amino substituents are strongly activating and ortho- and para-directing groups in electrophilic aromatic substitution reactions – Reactions are controlled by conversion to amide ...
Fulltext PDF
... Schrock carbene nucleophilic. Fischer carbenes have nearly opposite properties. The metal is electron rich, in part because of coordinate donation of an electron pair from the carbene carbon atom and CO ligands. Competition for the d-electrons of the metal takes place between bonding ligands such ...
... Schrock carbene nucleophilic. Fischer carbenes have nearly opposite properties. The metal is electron rich, in part because of coordinate donation of an electron pair from the carbene carbon atom and CO ligands. Competition for the d-electrons of the metal takes place between bonding ligands such ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.