Chapter 17. Aldehydes and Ketones
... reduction of carbonyl to methylene (Clemmensen or Wolff-Kishner) ...
... reduction of carbonyl to methylene (Clemmensen or Wolff-Kishner) ...
amine cured-epoxy matrices
... aromatic amines require elevated temperature to effectively cure most epoxy resins. It should also be mentioned that catalysts can be added to increase the reaction rate of amines with epoxy resins but if used in excess oftentimes at the cost of a reduction in performance. The amine curing agent sel ...
... aromatic amines require elevated temperature to effectively cure most epoxy resins. It should also be mentioned that catalysts can be added to increase the reaction rate of amines with epoxy resins but if used in excess oftentimes at the cost of a reduction in performance. The amine curing agent sel ...
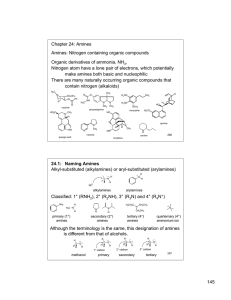
145 Chapter 24: Amines Amines: Nitrogen containing organic
... Alkyl ammonium ions, R3NH+ X-, have pKa values in the range of 10-11 (ammonium ion, H4N+ X-, has a pKa ~ 9.25) The ammonium ions of aryl amines and heterocyclic aromatic amines are considerably less basic than alkyl amines (pKa ~ 5 or less). The nitrogen lone pair is less basic if it is in an sp2 hy ...
... Alkyl ammonium ions, R3NH+ X-, have pKa values in the range of 10-11 (ammonium ion, H4N+ X-, has a pKa ~ 9.25) The ammonium ions of aryl amines and heterocyclic aromatic amines are considerably less basic than alkyl amines (pKa ~ 5 or less). The nitrogen lone pair is less basic if it is in an sp2 hy ...
ExamView Pro - Chapter 03.bnk
... tests on the macromolecules and collect the following information: 1) Test tubes #2 and #4 contain nitrogen, but the other tubes do not. 2) The contents of test tube #3 are not soluble in water, but the contents of the other test tubes are soluble in water. 3) The contents of test tube #1 can be bro ...
... tests on the macromolecules and collect the following information: 1) Test tubes #2 and #4 contain nitrogen, but the other tubes do not. 2) The contents of test tube #3 are not soluble in water, but the contents of the other test tubes are soluble in water. 3) The contents of test tube #1 can be bro ...
Topic 8 notes - A
... undergo addition are said to be unsaturated. Molecules which do not contain -bonds and which hence cannot undergo addition are said to be saturated. Alkenes are unsaturated and can hence undergo addition. Addition is the combination of two or more molecules to form a single molecule. Addition react ...
... undergo addition are said to be unsaturated. Molecules which do not contain -bonds and which hence cannot undergo addition are said to be saturated. Alkenes are unsaturated and can hence undergo addition. Addition is the combination of two or more molecules to form a single molecule. Addition react ...
ch221 class 5
... The terms are important because, although within a family, functional groups at 1o, 2o and 3o carbon atoms will have a similar chemistry, it will not be exactly the same for each. For example, 1-propanol (1o) may be more or less reactive in certain reactions than 2-propanol (2o). Naming Alkanes Triv ...
... The terms are important because, although within a family, functional groups at 1o, 2o and 3o carbon atoms will have a similar chemistry, it will not be exactly the same for each. For example, 1-propanol (1o) may be more or less reactive in certain reactions than 2-propanol (2o). Naming Alkanes Triv ...
Alcohols, phenols and ethers
... • Thioethers are organic compounds in which two saturated carbon atoms are linked through a single sulfur atom. • The common naming system for thioethers is similar to that for ethers, with the name “ether” being replaced by “sulfide” ...
... • Thioethers are organic compounds in which two saturated carbon atoms are linked through a single sulfur atom. • The common naming system for thioethers is similar to that for ethers, with the name “ether” being replaced by “sulfide” ...
Pharmacognosy-I (Part-7)
... 4. Reserve substances capable of supplying nitrogen or other elements necessary to the plant’s economy. ...
... 4. Reserve substances capable of supplying nitrogen or other elements necessary to the plant’s economy. ...
f8560d95306293b
... • The oxygen atom in alcohols, ethers and epoxides is sp3 hybridized. Alcohols and ethers have a bent shape like that in H2O. • The bond angle around the O atom in an alcohol or ether is similar to the tetrahedral bond angle of 109.5°. • Because the O atom is much more electronegative than carbon o ...
... • The oxygen atom in alcohols, ethers and epoxides is sp3 hybridized. Alcohols and ethers have a bent shape like that in H2O. • The bond angle around the O atom in an alcohol or ether is similar to the tetrahedral bond angle of 109.5°. • Because the O atom is much more electronegative than carbon o ...
CHEMISTRY 211 FINAL EXAM Wed., December 4, 2002 Name
... 13. (2.5) True or False? The pKa of a proton attached to the C of a terminal alkyne is more acidic than that in NH3. 14. (2.5) True or False? An achiral molecule must have a superimposable mirror image. 15. (4.5) For the following, circle all of the statements to the right which are correct. ...
... 13. (2.5) True or False? The pKa of a proton attached to the C of a terminal alkyne is more acidic than that in NH3. 14. (2.5) True or False? An achiral molecule must have a superimposable mirror image. 15. (4.5) For the following, circle all of the statements to the right which are correct. ...
from unt.edu - Department of Chemistry
... neutral polyfunctional inorganic Lewis acid (Hg3 (o-C6 F4 )3 ), and naked heavy metal cations (Tl+ and Ag+ ). The resulting sandwich adducts exhibit interesting bonding and optoelectronic properties. The focus of this article will be on the photophysical properties of the trinuclear Au(I) complexes ...
... neutral polyfunctional inorganic Lewis acid (Hg3 (o-C6 F4 )3 ), and naked heavy metal cations (Tl+ and Ag+ ). The resulting sandwich adducts exhibit interesting bonding and optoelectronic properties. The focus of this article will be on the photophysical properties of the trinuclear Au(I) complexes ...
CHEM 121. Chapter 15
... A) H atom from the alcohol bonds to the carbonyl carbon atom. B) OH portion from the alcohol bonds to the carbonyl oxygen atom. C) OR portion from the alcohol bonds to the carbonyl carbon atom. D) OH portion from the alcohol bonds to the carbonyl carbon atom. 18. A hemiacetal is a compound in which ...
... A) H atom from the alcohol bonds to the carbonyl carbon atom. B) OH portion from the alcohol bonds to the carbonyl oxygen atom. C) OR portion from the alcohol bonds to the carbonyl carbon atom. D) OH portion from the alcohol bonds to the carbonyl carbon atom. 18. A hemiacetal is a compound in which ...
UNSYMMETRICAL DINUCLEAR RHODIUM COMPLEXES WITH
... transition metal complexes of triorganoarsines which are efficient catalysts in organic synthesis are already known, the combination of an arsenic atom and one or more sulfur atoms in the same ligand is not widely exploited. Catalytic applications may be complicated by the fact that sulfur species a ...
... transition metal complexes of triorganoarsines which are efficient catalysts in organic synthesis are already known, the combination of an arsenic atom and one or more sulfur atoms in the same ligand is not widely exploited. Catalytic applications may be complicated by the fact that sulfur species a ...
Chapter 20 Organic Chemistry
... and other organisms, and alerts us to dangers such as polluted air or spoiled food. • Odorants must be volatile. • However, many volatile substances have no scent at all. • Most common smells are caused by organic molecules. • The study of compounds containing carbon combined with one or more of the ...
... and other organisms, and alerts us to dangers such as polluted air or spoiled food. • Odorants must be volatile. • However, many volatile substances have no scent at all. • Most common smells are caused by organic molecules. • The study of compounds containing carbon combined with one or more of the ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.