FREE Sample Here
... Full file at http://testbank360.eu/test-bank-organic-chemistry-7th-edition-leroy-g Organic Chemistry, 7e (Wade) Chapter 2 Structure and Properties of Organic Molecules 1) An orbital can be described by its __________, which is the mathematical description of the shape of the electron wave as it osci ...
... Full file at http://testbank360.eu/test-bank-organic-chemistry-7th-edition-leroy-g Organic Chemistry, 7e (Wade) Chapter 2 Structure and Properties of Organic Molecules 1) An orbital can be described by its __________, which is the mathematical description of the shape of the electron wave as it osci ...
chm121 tutorial kit - Covenant University
... The following are common drying agent for organic solutions but which of them has high capacity, fast speed, and good efficiency as its chemical property. (a) CaSO4 (b) CaCl 2 (c) K2CO3 (d) MgSO4 All except ------------- is not employed as an adsorbents in column chromatography (a) Starch (b) Silica ...
... The following are common drying agent for organic solutions but which of them has high capacity, fast speed, and good efficiency as its chemical property. (a) CaSO4 (b) CaCl 2 (c) K2CO3 (d) MgSO4 All except ------------- is not employed as an adsorbents in column chromatography (a) Starch (b) Silica ...
ALCOHOLS, PHENOLS AND ETHERS
... S o far you have learnt the chemistry of hydrocarbons which serve as basic skeleton for the attachment of various functional groups to give a large number of their derivatives. In the last lesson, we discussed one such class of compounds viz halogen derivatives of hydrocarbons. Another very useful a ...
... S o far you have learnt the chemistry of hydrocarbons which serve as basic skeleton for the attachment of various functional groups to give a large number of their derivatives. In the last lesson, we discussed one such class of compounds viz halogen derivatives of hydrocarbons. Another very useful a ...
Organic Chemistry Fifth Edition
... Very similar to opening of cyclic bromonium ion. Review that subject. Due to resonance, some positive charge is located on this carbon. ...
... Very similar to opening of cyclic bromonium ion. Review that subject. Due to resonance, some positive charge is located on this carbon. ...
Chapter 12
... KB 5 0S·4 H 20, Na2B4 0 7 ·10 H 20, CsB 20 4 , and Mg 3B7 0 13 Cl) give little idea of the structure of the anions in these substances. The main structural principles of the borates are similar to those for silicates: cyclic or linear polyoxo anions, formed by the linking together of B0 3 and/ or B0 ...
... KB 5 0S·4 H 20, Na2B4 0 7 ·10 H 20, CsB 20 4 , and Mg 3B7 0 13 Cl) give little idea of the structure of the anions in these substances. The main structural principles of the borates are similar to those for silicates: cyclic or linear polyoxo anions, formed by the linking together of B0 3 and/ or B0 ...
Document
... Benzene representation • Benzene representation howing a flat molecule with six delocalized electrons forming an cloud above and below the plane of the ring. • Properties of the benzene molecule and advanced bonding theory indicate this structure. The six electrons appear to be shared by all the ca ...
... Benzene representation • Benzene representation howing a flat molecule with six delocalized electrons forming an cloud above and below the plane of the ring. • Properties of the benzene molecule and advanced bonding theory indicate this structure. The six electrons appear to be shared by all the ca ...
Stereochemistry Tutorials: Assigning R/S and E/Z
... Definitions for vocabulary words can be found in the Illustrated Glossary of Organic Chemistry, available on the course web site. Discussion: Every organic compound needs an unambiguous name that clearly delineates all structural features of the molecule. The same is true for stereocenters. Because ...
... Definitions for vocabulary words can be found in the Illustrated Glossary of Organic Chemistry, available on the course web site. Discussion: Every organic compound needs an unambiguous name that clearly delineates all structural features of the molecule. The same is true for stereocenters. Because ...
3.2 Organic Synthesis (Reaction Pathways)
... In Higher Chemistry the alkylhalides were not particularly important chemicals. In Advanced Higher, however, the significance of halogenoalkanes (alkylhalides) cannot be overstressed. They can be a very important step in many Synthesis Pathways. ...
... In Higher Chemistry the alkylhalides were not particularly important chemicals. In Advanced Higher, however, the significance of halogenoalkanes (alkylhalides) cannot be overstressed. They can be a very important step in many Synthesis Pathways. ...
Alcohols
... inorganic oxidizing agents, such as KMnO4, CrO3, and Na2Cr2O7 Remember oxidation in Organic Chem refers to any reaction that adds bonds from carbon to oxygen and/or removes bonds from carbon to hydrogen ...
... inorganic oxidizing agents, such as KMnO4, CrO3, and Na2Cr2O7 Remember oxidation in Organic Chem refers to any reaction that adds bonds from carbon to oxygen and/or removes bonds from carbon to hydrogen ...
The First Chiral Organometallic Triangle for Asymmetric Catalysis
... NMR data of 1-4 showed a single ligand environment, suggesting the formation of cyclic species. For example, the 31P{1H} NMR spectrum of 1 exhibits a single peak at 3.51 ppm with a set of 195Pt satellites (JPt-P ) 2282.8 Hz). There is also only one single set of signals for the acetylenic and aromat ...
... NMR data of 1-4 showed a single ligand environment, suggesting the formation of cyclic species. For example, the 31P{1H} NMR spectrum of 1 exhibits a single peak at 3.51 ppm with a set of 195Pt satellites (JPt-P ) 2282.8 Hz). There is also only one single set of signals for the acetylenic and aromat ...
Alcohols, Phenols , Phenols and Ethers Alcohols
... and applications. Alcohols and phenols are formed when a hydrogen atom in a hydrocarbon, aliphatic and aromatic respectively, is replaced by –OH group. These classes of compounds find wide applications in industry as well as in day-to-day life. For instance, have you ever noticed that ordinary spiri ...
... and applications. Alcohols and phenols are formed when a hydrogen atom in a hydrocarbon, aliphatic and aromatic respectively, is replaced by –OH group. These classes of compounds find wide applications in industry as well as in day-to-day life. For instance, have you ever noticed that ordinary spiri ...
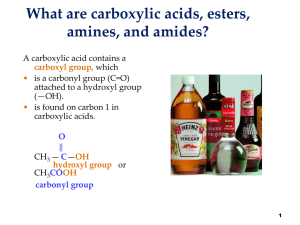
Esters amines and amides
... Solubility in water Amines • with 1-5 carbon atoms are soluble in water. • form hydrogen bonds with the polar O-H bond in water. ...
... Solubility in water Amines • with 1-5 carbon atoms are soluble in water. • form hydrogen bonds with the polar O-H bond in water. ...
FREE Sample Here
... D) larger size of molecules Answer: D Skill: Comprehension Objective: 2.6 33) What variable will determine if light passes through diamond, but not through graphite? A) the amount of protons present B) the arrangement of the electrons C) the arrangement of the nuclei D) the color of the different bo ...
... D) larger size of molecules Answer: D Skill: Comprehension Objective: 2.6 33) What variable will determine if light passes through diamond, but not through graphite? A) the amount of protons present B) the arrangement of the electrons C) the arrangement of the nuclei D) the color of the different bo ...
Lokshin2011
... from tricarbonyl complexes and dicarbonyl chelates are formed, stabilized by intramolecular coordination of the manganese atom with a substituent in the Cp-ring. This changes the color of the solution. In a closed system the CO molecules released during irradiation adds again to the intermediate and ...
... from tricarbonyl complexes and dicarbonyl chelates are formed, stabilized by intramolecular coordination of the manganese atom with a substituent in the Cp-ring. This changes the color of the solution. In a closed system the CO molecules released during irradiation adds again to the intermediate and ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.