stoker-C15
... (1) The compound 4-oxopentanal contains both an aldehyde and an ether functional group. (2) An alkoxy group and a hydroxy group attached to the same carbon atom are present in both hemiacetals and acetals. (3) Cyclic aldehyde structures are possible but cyclic ketone structures are not possible. A) ...
... (1) The compound 4-oxopentanal contains both an aldehyde and an ether functional group. (2) An alkoxy group and a hydroxy group attached to the same carbon atom are present in both hemiacetals and acetals. (3) Cyclic aldehyde structures are possible but cyclic ketone structures are not possible. A) ...
GENERAL INTRODUCTION TO THE CHEMISTRY OF DYES 1
... dyes that have affinity for protein substrates such as wool. Oxidation dyes, the more permanent of the two groups, are produced directly on the hair by oxidizing aromatic diamines such as para-phenylenediamine or 2,5-diaminotoluene with an oxidizing agent. Suitable diamines have been referred to as ...
... dyes that have affinity for protein substrates such as wool. Oxidation dyes, the more permanent of the two groups, are produced directly on the hair by oxidizing aromatic diamines such as para-phenylenediamine or 2,5-diaminotoluene with an oxidizing agent. Suitable diamines have been referred to as ...
研 究 業 績 リ ス ト
... (50) Ruthenium-Catalyzed Cycloaddition of Propargylic Alcohols with Phenol Derivatives via Allenylidene Intermediates: Catalytic Use of the Allenylidene Ligand as C3 Unit Y. Nishibayashi, Y. Inada, M. Hidai, S. Uemura J. Am. Chem. Soc., 124, 7900–7901 (2002). (51) Palladium(II) Complex-Catalysed Ena ...
... (50) Ruthenium-Catalyzed Cycloaddition of Propargylic Alcohols with Phenol Derivatives via Allenylidene Intermediates: Catalytic Use of the Allenylidene Ligand as C3 Unit Y. Nishibayashi, Y. Inada, M. Hidai, S. Uemura J. Am. Chem. Soc., 124, 7900–7901 (2002). (51) Palladium(II) Complex-Catalysed Ena ...
Section 2 Simple Molecular Orbital Theory
... form bonds. In all such qualitative mo analyses, the final results (i.e., how many mos there are of any given symmetry) will not depend on whether one thinks of the interactions involving atomic or hybrid orbitals. However, it is often easier to "guess" the bonding, non-bonding, and antibonding natu ...
... form bonds. In all such qualitative mo analyses, the final results (i.e., how many mos there are of any given symmetry) will not depend on whether one thinks of the interactions involving atomic or hybrid orbitals. However, it is often easier to "guess" the bonding, non-bonding, and antibonding natu ...
4.04 Nomenclature and Isomerism in Organic Chemistry
... Chiral molecules will rotate plane-polarised light. Two optical isomers will rotate plane polarised light equally, but in opposite directions. It is this difference in physical properties which enables them to be distinguished. It is not possible to predict the direction in which a particular optica ...
... Chiral molecules will rotate plane-polarised light. Two optical isomers will rotate plane polarised light equally, but in opposite directions. It is this difference in physical properties which enables them to be distinguished. It is not possible to predict the direction in which a particular optica ...
NOMENCLATURE OF ORGANIC COMPOUNDS - A
... Chiral molecules will rotate plane-polarised light. Two optical isomers will rotate plane polarised light equally, but in opposite directions. It is this difference in physical properties which enables them to be distinguished. It is not possible to predict the direction in which a particular optica ...
... Chiral molecules will rotate plane-polarised light. Two optical isomers will rotate plane polarised light equally, but in opposite directions. It is this difference in physical properties which enables them to be distinguished. It is not possible to predict the direction in which a particular optica ...
06. Alcohols. Phenols. Ethers
... II. In this common system, the position of an additional substituent is indicated by use of the Greek alphabet rather than by numbers. ...
... II. In this common system, the position of an additional substituent is indicated by use of the Greek alphabet rather than by numbers. ...
Ethers - Home - KSU Faculty Member websites
... students will: Know the structure of ethers Know the different methods of naming ethers Know the physical properties of ethers Know the different methods used in preparation of ethers Know the reactions of opened ethers with HX Know the different methods used in synthesis of epoxides Kno ...
... students will: Know the structure of ethers Know the different methods of naming ethers Know the physical properties of ethers Know the different methods used in preparation of ethers Know the reactions of opened ethers with HX Know the different methods used in synthesis of epoxides Kno ...
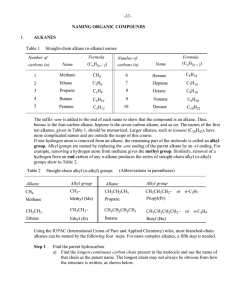
Naming Organic Compounds I
... ten alkanes, given in Table 1, should be memorized. Larger alkanes, such as icosane (C20H42), have more complicated names and are outside the scope of this course. If one hydrogen atom is removed from an alkane, the remaining part of the molecule is called an alkyl group. Alkyl groups are named by r ...
... ten alkanes, given in Table 1, should be memorized. Larger alkanes, such as icosane (C20H42), have more complicated names and are outside the scope of this course. If one hydrogen atom is removed from an alkane, the remaining part of the molecule is called an alkyl group. Alkyl groups are named by r ...
Chapter 7. Alcohols, Thiols, Phenols, Ethers
... Acidic properties of alcohols The high electronegativity of the oxygen atom in alcohols causes the oxygen to take electrons away from the hydrogen atom. The OH bond is polarized and is able to give up a proton, H+ . Thus alcohols are weak acids, but can become stronger acids depending on the types o ...
... Acidic properties of alcohols The high electronegativity of the oxygen atom in alcohols causes the oxygen to take electrons away from the hydrogen atom. The OH bond is polarized and is able to give up a proton, H+ . Thus alcohols are weak acids, but can become stronger acids depending on the types o ...
Introduction - St. Olaf College
... shifts, select Properties from the Display menu (to bring up the Atom Properties dialog) and click on the atom of interest. Note that proton shifts in addition to 13C shifts are available. ...
... shifts, select Properties from the Display menu (to bring up the Atom Properties dialog) and click on the atom of interest. Note that proton shifts in addition to 13C shifts are available. ...
Chapter 20 Organic Chemistry
... models not only show you the attachment pattern, but give you an idea about the shape of the molecule ...
... models not only show you the attachment pattern, but give you an idea about the shape of the molecule ...
Chemical Properties of Aldehydes and Ketones
... Similar reactions can occur at the other two reactive sites on each phenol molecule, leading to the formation of the polymer. This polymer is thermosetting because it has an extensively cross-linked network structure. ...
... Similar reactions can occur at the other two reactive sites on each phenol molecule, leading to the formation of the polymer. This polymer is thermosetting because it has an extensively cross-linked network structure. ...
The Grob Fragmentation
... -Grob fragmentation: Fragmentation substrates are typically 1,3diheterofunctionalized compounds featuring a nucelophilic atom with a negative ...
... -Grob fragmentation: Fragmentation substrates are typically 1,3diheterofunctionalized compounds featuring a nucelophilic atom with a negative ...
IUPAC System of Nomenclature
... CH3 CH3 CH2 CHCH2 CH2 CH3 is regarded as being "made" from the following parent: ...
... CH3 CH3 CH2 CHCH2 CH2 CH3 is regarded as being "made" from the following parent: ...
Chapter 17 Amines
... (1857-1935). Sandmeyer joined Geigy as a research scientist in 1888, and eventually became a director of the firm. He discovered the decomposition of aryl diazonium Chlorides to chloroarenes in the presence of copper (I) chloride in 1884. He also worked on the triphenylmethane dyes and the synthesis ...
... (1857-1935). Sandmeyer joined Geigy as a research scientist in 1888, and eventually became a director of the firm. He discovered the decomposition of aryl diazonium Chlorides to chloroarenes in the presence of copper (I) chloride in 1884. He also worked on the triphenylmethane dyes and the synthesis ...
CHAPTER 21 ORGANIC CHEMISTRY
... with 120° bond angles. Each carbon is sp2 hybridized. The sp2 hybrid orbitals go to form the three sigma bonds to each carbon. The unhybridized p atomic orbitals on each carbon overlap side to side with unhybridized p orbitals on adjacent carbons to form the π bonds. All six of the carbons in the si ...
... with 120° bond angles. Each carbon is sp2 hybridized. The sp2 hybrid orbitals go to form the three sigma bonds to each carbon. The unhybridized p atomic orbitals on each carbon overlap side to side with unhybridized p orbitals on adjacent carbons to form the π bonds. All six of the carbons in the si ...
ch01
... a place where is negative. a place where is positive. a place where = 0. a place where 2 is large. a place where 2 is negative. Ans: C ...
... a place where is negative. a place where is positive. a place where = 0. a place where 2 is large. a place where 2 is negative. Ans: C ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.