Chapter 9 Modified
... glucose NADH electron transport chain proton-motive force ATP • About 34% of the energy in a glucose molecule is transferred to ATP during cellular respiration, making about 32 ATP • There are several reasons why the number of ATP is not known exactly © 2011 Pearson Education, Inc. ...
... glucose NADH electron transport chain proton-motive force ATP • About 34% of the energy in a glucose molecule is transferred to ATP during cellular respiration, making about 32 ATP • There are several reasons why the number of ATP is not known exactly © 2011 Pearson Education, Inc. ...
second exam2
... the maximum possible membrane potential that could be generated by NADH oxidation by oxygen and the maximum amount of ATP that could be generated from this process. ALL WORK MUST BE SHOWN FOR ANY CREDIT. a) 5 points. Consider the oxidation of NADH by oxygen (this is the reaction run in your body to ...
... the maximum possible membrane potential that could be generated by NADH oxidation by oxygen and the maximum amount of ATP that could be generated from this process. ALL WORK MUST BE SHOWN FOR ANY CREDIT. a) 5 points. Consider the oxidation of NADH by oxygen (this is the reaction run in your body to ...
C nuclear magnetic resonance studies of anaerobic
... Embden-Meyerhof scheme, first proposed by Grant and Fulton (1$). Under anaerobic conditions however, when equimolar quantities of pyruvate and glycerol are produced (12,14), reservations have been expressed as to the validity of this pathway (15,16). Aerobically, pyruvate is considered to be the exc ...
... Embden-Meyerhof scheme, first proposed by Grant and Fulton (1$). Under anaerobic conditions however, when equimolar quantities of pyruvate and glycerol are produced (12,14), reservations have been expressed as to the validity of this pathway (15,16). Aerobically, pyruvate is considered to be the exc ...
[edit] Amino acids and proteins [edit] Lipids
... through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy and will not occur by themselves, by coupling them to spontaneous reactions that release energy. As enzymes act a ...
... through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy and will not occur by themselves, by coupling them to spontaneous reactions that release energy. As enzymes act a ...
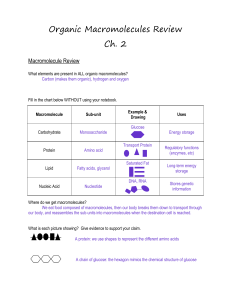
Organic Macromolecules Review Ch. 2
... (enzymes, etc) Long-term energy storage Stores genetic information ...
... (enzymes, etc) Long-term energy storage Stores genetic information ...
Ch18.doc
... dehydrogenase (1 NADH) and one turn of the CAC: yielding 3NADH, 1FADH2 and 1 GTP. Converting NADH and FADH2 to ATPs we use 1 NADH = 2.5 ATP and 1 FADH2 = 1.5 ATP. So for one alanine: (3+1)(2.5 ATP) + 1.5 ATP + 1 ATP = 12.5 ATP. But now it will cost some ATP to get rid of the amino group: so it would ...
... dehydrogenase (1 NADH) and one turn of the CAC: yielding 3NADH, 1FADH2 and 1 GTP. Converting NADH and FADH2 to ATPs we use 1 NADH = 2.5 ATP and 1 FADH2 = 1.5 ATP. So for one alanine: (3+1)(2.5 ATP) + 1.5 ATP + 1 ATP = 12.5 ATP. But now it will cost some ATP to get rid of the amino group: so it would ...
Honors Biology Name Biochemistry Exam Review #1 Period _____
... The material an enzyme works on is called the substrates. The pocket or groove where the substrate fits into on the enzyme is called the active site. (See diagram in enzyme notes for enzyme structure) Enzymes are named for the substrate that they work with. Names usually end in –ase (ex. Lactase, He ...
... The material an enzyme works on is called the substrates. The pocket or groove where the substrate fits into on the enzyme is called the active site. (See diagram in enzyme notes for enzyme structure) Enzymes are named for the substrate that they work with. Names usually end in –ase (ex. Lactase, He ...
(Semester VI) Paper 15: PLANT METABOLISM THEORY Unit 1
... Unit 3: Carbohydrate metabolism Synthesis and catabolism of sucrose and starch. ...
... Unit 3: Carbohydrate metabolism Synthesis and catabolism of sucrose and starch. ...
1 - Chiropractic National Board Review Questions
... 84. What nutrients form a coenzyme which is used directly for amino acid Transamination? A. Pyroxine 85. What is catecholamine synthesized from? A. Epinephrine 86. How many essential amino acids are aromatic? A. 2 87. Thyroxime is derived from? A. Threonine B. Tyrosine C. Tyramine D. Thiamine 88. En ...
... 84. What nutrients form a coenzyme which is used directly for amino acid Transamination? A. Pyroxine 85. What is catecholamine synthesized from? A. Epinephrine 86. How many essential amino acids are aromatic? A. 2 87. Thyroxime is derived from? A. Threonine B. Tyrosine C. Tyramine D. Thiamine 88. En ...
Pyruvate Dehydrogenase
... Under the same conditions, the amount of Pyruvate Dehydrogenase Phosphatase decreases. The resulting inhibition of Pyruvate Dehydrogenase prevents muscle and other tissues from catabolizing glucose ...
... Under the same conditions, the amount of Pyruvate Dehydrogenase Phosphatase decreases. The resulting inhibition of Pyruvate Dehydrogenase prevents muscle and other tissues from catabolizing glucose ...
Name_______________________________
... A. They increase the energy that is released by reactions. B. They increase the energy that must be absorbed by reactions. C. They are not used up by reactions. D. They must be continuously replaced after each catalyzed reaction. ...
... A. They increase the energy that is released by reactions. B. They increase the energy that must be absorbed by reactions. C. They are not used up by reactions. D. They must be continuously replaced after each catalyzed reaction. ...
BIO 10 Lecture 2
... Glycolysis: • Glucose enters the cytoplasm of the cell • An enzyme immediately breaks apart one ATP molecule into ADP and P (produces energy) – The phosphate (P) group in then attached to the glucose, creating glucose-6-phosphate ...
... Glycolysis: • Glucose enters the cytoplasm of the cell • An enzyme immediately breaks apart one ATP molecule into ADP and P (produces energy) – The phosphate (P) group in then attached to the glucose, creating glucose-6-phosphate ...
03-232 Biochemistry Exam III - S2014 Name:________________________
... 13. (2 pts) The maximum yield of ethanol from glucose will be obtained under conditions of high or low oxygen content? (circle correct answer). [Under conditions of low oxygen, NAD+ cannot be regenerated by electron transport. The conversion of pyruvate to ethanol oxidizes NADH back to NAD +.] 14. ( ...
... 13. (2 pts) The maximum yield of ethanol from glucose will be obtained under conditions of high or low oxygen content? (circle correct answer). [Under conditions of low oxygen, NAD+ cannot be regenerated by electron transport. The conversion of pyruvate to ethanol oxidizes NADH back to NAD +.] 14. ( ...
Skills Worksheet
... via photosynthesis and other metabolic processes and by breaking organic compounds down via cellular respiration and other metabolic processes. In these ways, organisms contribute to the cycling of carbon through the environment. 24. Autotrophs are able to use energy from sunlight to make organic co ...
... via photosynthesis and other metabolic processes and by breaking organic compounds down via cellular respiration and other metabolic processes. In these ways, organisms contribute to the cycling of carbon through the environment. 24. Autotrophs are able to use energy from sunlight to make organic co ...
Chapter 3
... against the effectors of human sleeping sickness and the animal trypanosomiasis; T . b . b r u c e i, T .b e v a n s i and T . b . e q u i p e r i d u m . M e l a r s o p r o l is still the drug of choice in late stage trypanosomiasis. It is, however, associated with severe side effects, which are o ...
... against the effectors of human sleeping sickness and the animal trypanosomiasis; T . b . b r u c e i, T .b e v a n s i and T . b . e q u i p e r i d u m . M e l a r s o p r o l is still the drug of choice in late stage trypanosomiasis. It is, however, associated with severe side effects, which are o ...
Question Report - FM Faculty Web Pages
... states that germs are the fundamental units of life states that germs can invade other organisms and cause disease states that germs will spontaneously arise from decaying meat is another name for the cell theory ...
... states that germs are the fundamental units of life states that germs can invade other organisms and cause disease states that germs will spontaneously arise from decaying meat is another name for the cell theory ...
BTEC National Unit 1 Energy Systems KW version
... If the activity is short in duration (less than 10 seconds) and high intensity, we use the ATP-PC system If the activity is longer than 10 seconds and up to 3 minutes at high intensity, we use the lactic acid system If the activity is long in duration and submaximal pace, we use the aerobic sy ...
... If the activity is short in duration (less than 10 seconds) and high intensity, we use the ATP-PC system If the activity is longer than 10 seconds and up to 3 minutes at high intensity, we use the lactic acid system If the activity is long in duration and submaximal pace, we use the aerobic sy ...
Work Physiology
... Glucose → pyrovic acid + 2 ATP Pyrovic acid → acetyl coA + H2O + CO2 Acetyl coA → CO2 + H + 2 ATP (Krebs cycle) Oxidation of hydrogens (oxidative phosphorylation): 30 ATP 1 mole glucose: 686000 calories 1 mole glucose: 38 ATP (456000 calories) ...
... Glucose → pyrovic acid + 2 ATP Pyrovic acid → acetyl coA + H2O + CO2 Acetyl coA → CO2 + H + 2 ATP (Krebs cycle) Oxidation of hydrogens (oxidative phosphorylation): 30 ATP 1 mole glucose: 686000 calories 1 mole glucose: 38 ATP (456000 calories) ...
Photosynthesis Modeling Activity
... cellulose, which are polymers of glucose. Other glucose molecules go on to cellular respiration which creates useable energy for the cells (ATP) from glucose. The sugars produced by photosynthesis are also used to make other plant molecules such as the amino acids which are the building blocks for p ...
... cellulose, which are polymers of glucose. Other glucose molecules go on to cellular respiration which creates useable energy for the cells (ATP) from glucose. The sugars produced by photosynthesis are also used to make other plant molecules such as the amino acids which are the building blocks for p ...
Acetyl CoA
... yields D-bhydroxybutyrate (do not confuse with L- bhydroxybutyrate of the boxidation pathway). 5. Acetoacetate is easily decarboxylated (may be spontaneously or enzymatically) to acetone and CO2. ...
... yields D-bhydroxybutyrate (do not confuse with L- bhydroxybutyrate of the boxidation pathway). 5. Acetoacetate is easily decarboxylated (may be spontaneously or enzymatically) to acetone and CO2. ...
Electron Transport Chain, Oxidative phosphorylation and Pentose
... 18. Boyer’s hypothesis was partly true and it is applied in oxidative phophorylation. What are the steps where it is applied correctly? Indeed, Boyer’s theory of conformational change was true for explaining Proton pumping stage (Complex I, III and IV) and also for ATP synthesis by F1. 19. How many ...
... 18. Boyer’s hypothesis was partly true and it is applied in oxidative phophorylation. What are the steps where it is applied correctly? Indeed, Boyer’s theory of conformational change was true for explaining Proton pumping stage (Complex I, III and IV) and also for ATP synthesis by F1. 19. How many ...
1a ExamI Intro-MicrGrwth
... order to get reliable gram staining results? a. 0-1 hours b. 1-3 hours c. 3-5 hours d. 5-7 hours e. Any time would work equally well 25. Which of the following end products are formed by yeast performing fermentation? a. pyruvate b. water c. lactic acid d. ethanol and CO2 e. 34-38 ATP per glucose 26 ...
... order to get reliable gram staining results? a. 0-1 hours b. 1-3 hours c. 3-5 hours d. 5-7 hours e. Any time would work equally well 25. Which of the following end products are formed by yeast performing fermentation? a. pyruvate b. water c. lactic acid d. ethanol and CO2 e. 34-38 ATP per glucose 26 ...
Answer Key - Test Banks Shop
... 8. Complex molecules are broken down and nutrients are absorbed into the bloodstream for transport to tissue cells by the _______________. A) digestive system B) liver C) kidneys D) endocrine system 9. The liver has many functions. Which one of the following is NOT a function of the liver? A) produc ...
... 8. Complex molecules are broken down and nutrients are absorbed into the bloodstream for transport to tissue cells by the _______________. A) digestive system B) liver C) kidneys D) endocrine system 9. The liver has many functions. Which one of the following is NOT a function of the liver? A) produc ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑


![[edit] Amino acids and proteins [edit] Lipids](http://s1.studyres.com/store/data/017606867_1-0f8e8f7866b15e60475e6df20c71fc0c-300x300.png)