Aerobic Metabolism ii: electron transport chain
... The cells of all eukaryotes (all animals, plants, fungi, algae – in other words, all living things except bacteria and archaea) contain intracellular organelles called mitochondria that produce ATP. Energy sources such as glucose are initially metabolized in the cytoplasm. The products are imported ...
... The cells of all eukaryotes (all animals, plants, fungi, algae – in other words, all living things except bacteria and archaea) contain intracellular organelles called mitochondria that produce ATP. Energy sources such as glucose are initially metabolized in the cytoplasm. The products are imported ...
Part 1 - ISpatula
... – Kingdom Plantae – Kingdom Animalia – Kingdom Fungi – Kingdom Bacteria • These processes demonstrate the fundamental unity of all living matter, and are collectively described as primary metabolism, with the compounds involved in the pathways being termed primary metabolites ...
... – Kingdom Plantae – Kingdom Animalia – Kingdom Fungi – Kingdom Bacteria • These processes demonstrate the fundamental unity of all living matter, and are collectively described as primary metabolism, with the compounds involved in the pathways being termed primary metabolites ...
REVIEW SHEET FOR EVOLUTION UNIT
... In which process is glucose broken down in cells and ATP produced? What gas is required for aerobic respiration to occur and where does this process occur in the cell? What is the difference between oxidation and reduction? How many oxygen atoms are needed to fully oxidize or breakdown sugar? (look ...
... In which process is glucose broken down in cells and ATP produced? What gas is required for aerobic respiration to occur and where does this process occur in the cell? What is the difference between oxidation and reduction? How many oxygen atoms are needed to fully oxidize or breakdown sugar? (look ...
Artificial Photosynthesis - The Mars Homestead Project
... located on the outer surface of the thylakoid membrane. 2. Mg+2, which enters the stroma as H+ ions leave when chloroplasts are illuminated. 3. NADPH, which is generated by photosystem I during illumination. CO2 fixation is a dark reaction, but it is regulated by the light reaction ...
... located on the outer surface of the thylakoid membrane. 2. Mg+2, which enters the stroma as H+ ions leave when chloroplasts are illuminated. 3. NADPH, which is generated by photosystem I during illumination. CO2 fixation is a dark reaction, but it is regulated by the light reaction ...
Biosynthesis of Nucleotides 1 - University of Alabama at Birmingham
... Rapidly growing cells, such as infective bacteria and fast-growing tumors, are more susceptible to such agents Sulfonamides are effective antibacterial agents Methotrexate and aminopterin are folic acid analogs that have been used in cancer chemotherapy ...
... Rapidly growing cells, such as infective bacteria and fast-growing tumors, are more susceptible to such agents Sulfonamides are effective antibacterial agents Methotrexate and aminopterin are folic acid analogs that have been used in cancer chemotherapy ...
Thermodynamic considerations of carbon dioxide evolution in
... the present study are as follows: is (i) The reaction, pathway 'H + HCO; - > COzo,,close to equilibrium, since the free energy change of the overall process is only a fraction of 1 kcal mol-'. This value may be compared to the free energy change of the glycolytic pathway in human erythrocytes (-25.2 ...
... the present study are as follows: is (i) The reaction, pathway 'H + HCO; - > COzo,,close to equilibrium, since the free energy change of the overall process is only a fraction of 1 kcal mol-'. This value may be compared to the free energy change of the glycolytic pathway in human erythrocytes (-25.2 ...
Oxidative phosphorylation RESP312
... The electrons finally combine with O2 and proton (H+) to form H2O. This requirement for O2 makes the electron transport process the respiratory chain . ...
... The electrons finally combine with O2 and proton (H+) to form H2O. This requirement for O2 makes the electron transport process the respiratory chain . ...
ETC_2012 Quiz
... Q. More negative the Eo _______________ to lose electrons • More positive the Eo greater the potential to accept electrons • Electrons therefore flow from the pair with the more negative Eo to the most positive one • The order of the complexes in the ETC is from the more negative to more positive ...
... Q. More negative the Eo _______________ to lose electrons • More positive the Eo greater the potential to accept electrons • Electrons therefore flow from the pair with the more negative Eo to the most positive one • The order of the complexes in the ETC is from the more negative to more positive ...
electron transport chain
... transport chain. Electron transport chains produce energy in the form of a transmembrane electrochemical potential gradient. This energy is used to do useful work. The gradient can be used to transport molecules across membranes. It can be used to produce ATP and NADH, high-energy molecules that are ...
... transport chain. Electron transport chains produce energy in the form of a transmembrane electrochemical potential gradient. This energy is used to do useful work. The gradient can be used to transport molecules across membranes. It can be used to produce ATP and NADH, high-energy molecules that are ...
Biology 3A Exam 2 Study Guide The exam will consist of multiple
... • What is the fate of the foodstuff that we eat if they are not burned for energy? • compare and contrast chemiosmosis in the mitochondrion and chloroplast Photosynthesis - where does it take place, reactants, products • How do plants capture light energy? Your answer should include the following te ...
... • What is the fate of the foodstuff that we eat if they are not burned for energy? • compare and contrast chemiosmosis in the mitochondrion and chloroplast Photosynthesis - where does it take place, reactants, products • How do plants capture light energy? Your answer should include the following te ...
S1 Text Section A Annotation by structural analysis In case of aldose
... model lethal predictions and their relation to known phenotypes identified from experiments. The results suggest that the iAS142 model can accurately predict the actual phenotypes identified through known knockout experiments in comparison to iAC560. These differences are primarily due to difference ...
... model lethal predictions and their relation to known phenotypes identified from experiments. The results suggest that the iAS142 model can accurately predict the actual phenotypes identified through known knockout experiments in comparison to iAC560. These differences are primarily due to difference ...
protein - Humble ISD
... A student performed several chemical tests of 4 unknown foods. The results are given below. Which unknown is most likely from egg white and why? ...
... A student performed several chemical tests of 4 unknown foods. The results are given below. Which unknown is most likely from egg white and why? ...
Cellular Respiration and Fermentation
... how is it regulated? Then take a look in the mirror. All these processes are occurring right now, in virtually all your cells. ...
... how is it regulated? Then take a look in the mirror. All these processes are occurring right now, in virtually all your cells. ...
Homeostasis - Cloudfront.net
... • an oxygen dissociation curve shows the degree of hemoglobin saturation with oxygen plotted against different values of p(O2) – the curve is Sshaped • at p(O2) close to zero there is no oxygen bound to the hemoglobin • at low p(O2), the polypeptide chains are tightly bound together, making it diff ...
... • an oxygen dissociation curve shows the degree of hemoglobin saturation with oxygen plotted against different values of p(O2) – the curve is Sshaped • at p(O2) close to zero there is no oxygen bound to the hemoglobin • at low p(O2), the polypeptide chains are tightly bound together, making it diff ...
Lecture-Oxidative Phsphorylation
... Oxidative Phosphorylation What is mitochondria? 2 membranes: Inner - only permeable to O2, H2O transporters req’d for ATP, Pi, pyruvate, etc. folding increases surface area (site of ox. phos. machinery) Matrix contains: citric acid cycle enzymes Fatty acid oxidation enzymes (discuss later) ...
... Oxidative Phosphorylation What is mitochondria? 2 membranes: Inner - only permeable to O2, H2O transporters req’d for ATP, Pi, pyruvate, etc. folding increases surface area (site of ox. phos. machinery) Matrix contains: citric acid cycle enzymes Fatty acid oxidation enzymes (discuss later) ...
cellrespiration power pointtext
... – Can produce ATP with or without oxygen, in aerobic or anaerobic conditions – Couples with fermentation to produce ATP ...
... – Can produce ATP with or without oxygen, in aerobic or anaerobic conditions – Couples with fermentation to produce ATP ...
in the fatty acid
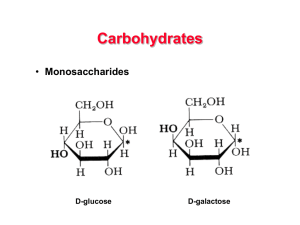
... – below – alpha form – starch – all glucoses are in the alpha form – cellulose – all glucoses are in the beta form – which makes every other glucose upside down (a) and glucose ring structures ...
... – below – alpha form – starch – all glucoses are in the alpha form – cellulose – all glucoses are in the beta form – which makes every other glucose upside down (a) and glucose ring structures ...
CHAPTERS 2 & 3 Continued
... – Glucose and fructose are six carbons long – Others have three to seven carbon atoms ...
... – Glucose and fructose are six carbons long – Others have three to seven carbon atoms ...
QUIZ #7 NUCLEOTIDE METABOLISM
... a. is utilized in the synthesis of the purine ring b. is required for the salvage of purine bases c. converts hypoxanthine to guanine d. is inhibited by methotrexate e. is required to convert purines to uric acid ...
... a. is utilized in the synthesis of the purine ring b. is required for the salvage of purine bases c. converts hypoxanthine to guanine d. is inhibited by methotrexate e. is required to convert purines to uric acid ...
Bacterial cultivation - Furry Helpers Pet Sitting
... Carbohydrates are sugars and they serve as energy source for bacteria ...
... Carbohydrates are sugars and they serve as energy source for bacteria ...
Carbohydrates
... proinsulin (A, B and C peptides) insulin + C-peptide » anabolic (synthesis) » promotes cellular uptake of glucose » increased: • lipogenesis • protein synthesis • glycogenesis » decreased: • lipolysis • ketone formation • gluconeogenesis • glycogenolysis ...
... proinsulin (A, B and C peptides) insulin + C-peptide » anabolic (synthesis) » promotes cellular uptake of glucose » increased: • lipogenesis • protein synthesis • glycogenesis » decreased: • lipolysis • ketone formation • gluconeogenesis • glycogenolysis ...
Lecture 5
... The Free-Energy Change for a Reaction Determines Whether It Can Occur Although enzymes speed up reactions, they cannot by themselves force energetically unfavorable reactions to occur. According to the second law of thermodynamics, a chemical reaction can proceed only if it results in net increase ...
... The Free-Energy Change for a Reaction Determines Whether It Can Occur Although enzymes speed up reactions, they cannot by themselves force energetically unfavorable reactions to occur. According to the second law of thermodynamics, a chemical reaction can proceed only if it results in net increase ...
Anaerobic yeast fermentation for the production of ethanol in a
... Anaerobic yeast fermentation for the production of ethanol in a versatile lab fermentor Whether used for research or production, the versatile BioFlo® 310 fermentor from New Brunswick Scientific allows growth of a wide variety of aerobic and anaerobic microorganisms, including bacteria, plant, algae ...
... Anaerobic yeast fermentation for the production of ethanol in a versatile lab fermentor Whether used for research or production, the versatile BioFlo® 310 fermentor from New Brunswick Scientific allows growth of a wide variety of aerobic and anaerobic microorganisms, including bacteria, plant, algae ...
Bio Chem webquest
... 11. Describe how glycolysis and photosynthesis are related. 12. Why do you need to build molecules? 13. What is ATP? Click on 14. What IS a carbohydrate? 15. What is the function of a carbohydrate? 16. What is a monosaccharide? 17. What is a disaccharide? 18. What is a polysaccharide? 19. What is gl ...
... 11. Describe how glycolysis and photosynthesis are related. 12. Why do you need to build molecules? 13. What is ATP? Click on 14. What IS a carbohydrate? 15. What is the function of a carbohydrate? 16. What is a monosaccharide? 17. What is a disaccharide? 18. What is a polysaccharide? 19. What is gl ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑