Methods for Determining the Biochemical Activities of Micro
... toxins whose modes of action have been elucidated have been shown to be enzymes and there is no reason to suppose that collagenase and hyaluronidase are any less or more important than urease or glutamic acid decarboxylase. Even the demonstration of morphological features depends on the presence of ...
... toxins whose modes of action have been elucidated have been shown to be enzymes and there is no reason to suppose that collagenase and hyaluronidase are any less or more important than urease or glutamic acid decarboxylase. Even the demonstration of morphological features depends on the presence of ...
Molecules of Life Powerpoint
... Functional Groups • Groups of atoms known as functional groups can give special properties on carbon-based molecules. • Carbon is a central element to life because most biological molecules are built on a carbon framework. ...
... Functional Groups • Groups of atoms known as functional groups can give special properties on carbon-based molecules. • Carbon is a central element to life because most biological molecules are built on a carbon framework. ...
Chapter 3
... – Lactate is an important ________________. – Active muscles produce and use lacate at rest and during exercise. – Lactate that is produced as an end-product of glycolysis is • transported into _______________oxidized via Krebs cycle • Released into the blood stream where it can be used by other mus ...
... – Lactate is an important ________________. – Active muscles produce and use lacate at rest and during exercise. – Lactate that is produced as an end-product of glycolysis is • transported into _______________oxidized via Krebs cycle • Released into the blood stream where it can be used by other mus ...
Chapter 1 • Lesson 4 Objectives 4
... Most catalysts of biochemical reactions are enzymes. Recall that an enzyme is a protein that serves as a catalyst, enabling or speeding chemical reactions in cells. It makes those reactions possible, or speeds them up, without being changed by the reaction. Without enzymes, many of the chemical reac ...
... Most catalysts of biochemical reactions are enzymes. Recall that an enzyme is a protein that serves as a catalyst, enabling or speeding chemical reactions in cells. It makes those reactions possible, or speeds them up, without being changed by the reaction. Without enzymes, many of the chemical reac ...
Journal of Biotechnology Evaluation of 13C isotopic tracers for
... determines the confidence of flux estimates, which are as important as the flux values themselves (Antoniewicz et al., 2006). Stationary MFA is conducted when the labeled substrate is at isotopic steady state and does not utilize pool size or transient data; as such, this technique is especially relian ...
... determines the confidence of flux estimates, which are as important as the flux values themselves (Antoniewicz et al., 2006). Stationary MFA is conducted when the labeled substrate is at isotopic steady state and does not utilize pool size or transient data; as such, this technique is especially relian ...
Slid 7 Hops
... clock wise (1-10). We have different derivatives, one is Anthraquinone (which has a keto groups at carbons no. 9,10), if the keto group at carbon number 10 was removed then its Anthrone, if carbon number 10 was oxidized we will have Oxanthrone, and when carbon number 9 has a phenolic group (reductio ...
... clock wise (1-10). We have different derivatives, one is Anthraquinone (which has a keto groups at carbons no. 9,10), if the keto group at carbon number 10 was removed then its Anthrone, if carbon number 10 was oxidized we will have Oxanthrone, and when carbon number 9 has a phenolic group (reductio ...
Exercise Physiology
... Responses to Anaerobic Exercise • In order to immediately meet the sudden higher energy demand, stored ATP is the first energy source. This lasts for approximately 2 seconds. • When stored ATP is used up the ATP-PC system kicks in but it can only last 8-10 seconds before PC stores are depleted. • Th ...
... Responses to Anaerobic Exercise • In order to immediately meet the sudden higher energy demand, stored ATP is the first energy source. This lasts for approximately 2 seconds. • When stored ATP is used up the ATP-PC system kicks in but it can only last 8-10 seconds before PC stores are depleted. • Th ...
February 5 AP Biology - John D. O`Bryant School of Math & Science
... Unlike in cellular respiration, the proton motive force generated by the light reactions in photosynthesis happens in three ways… Can you remember the three ...
... Unlike in cellular respiration, the proton motive force generated by the light reactions in photosynthesis happens in three ways… Can you remember the three ...
Scheme of work for Option C, Cells and energy
... Copyright Cambridge University Press 2011. All rights reserved. ...
... Copyright Cambridge University Press 2011. All rights reserved. ...
Slide 1
... acids in the blood in order to oxidize them for energy production • Adipose tissue contains hormone-sensitive lipase (HSL), that is activated by PKAdependent phosphorylation leading to increased fatty acid release to the blood ...
... acids in the blood in order to oxidize them for energy production • Adipose tissue contains hormone-sensitive lipase (HSL), that is activated by PKAdependent phosphorylation leading to increased fatty acid release to the blood ...
Application of stable isotopes and mass isotopomer distribution
... after chemical or enzymatic cleavage to yield specific molecular portions (e.g., glucose), the methods are labor intensive and for some molecules (e.g., [U-14C] glycerol) it may not be possible. Consequently, use of certain radiolabeled compounds may limit information on the flux through and activit ...
... after chemical or enzymatic cleavage to yield specific molecular portions (e.g., glucose), the methods are labor intensive and for some molecules (e.g., [U-14C] glycerol) it may not be possible. Consequently, use of certain radiolabeled compounds may limit information on the flux through and activit ...
Enzymes I
... Hexokinase and glucokinase phosphorylate glucose; hemokinase has small Km therefore high affinity and much quicker; opposite is true for glucokinase in liver Multisubstrate reactions: If two substrates are required for rxn to procedd, both has influence on curve Serine Proteases o a family of enzyme ...
... Hexokinase and glucokinase phosphorylate glucose; hemokinase has small Km therefore high affinity and much quicker; opposite is true for glucokinase in liver Multisubstrate reactions: If two substrates are required for rxn to procedd, both has influence on curve Serine Proteases o a family of enzyme ...
VCE PE Unit 3: Preparing Students for the End of Year Exam
... • However, which energy system/s is predominate depends upon the ATP demand of the activity. • Two factors determine ATP demand: • Exercise duration – how long the activity lasts for • Exercise intensity – how hard the exercise is ...
... • However, which energy system/s is predominate depends upon the ATP demand of the activity. • Two factors determine ATP demand: • Exercise duration – how long the activity lasts for • Exercise intensity – how hard the exercise is ...
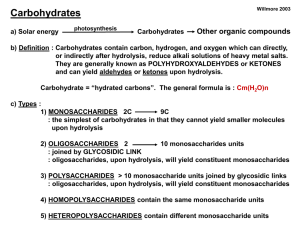
Karbohidratlar - mustafaaltinisik.org.uk
... - nucleophilic attack of phosphate on C1 - chopping off at non reducing end (branching points are obstacles) - phosphorilytic degradation of glycogen results in release of D-glucose-1-phosphate - controlled by consumption of the product - enzyme phosphorylase regulated and this system is crutial i ...
... - nucleophilic attack of phosphate on C1 - chopping off at non reducing end (branching points are obstacles) - phosphorilytic degradation of glycogen results in release of D-glucose-1-phosphate - controlled by consumption of the product - enzyme phosphorylase regulated and this system is crutial i ...
Glycogen Earth organisms use three major forms of - Rose
... glucose units. Because glucose can readily be both added and removed from glycogen, glycogen acts as a useful storage form for glucose. In principle, it would be possible to store free glucose; in practice storing glucose would result in severe complications due to the much higher osmotic pressure o ...
... glucose units. Because glucose can readily be both added and removed from glycogen, glycogen acts as a useful storage form for glucose. In principle, it would be possible to store free glucose; in practice storing glucose would result in severe complications due to the much higher osmotic pressure o ...
Pharmaceutical Faculty 3- d course Module 1 General principles of
... C. Conversion of fat and/ or protein to glucose D. The anaerobic metabolism of glucose E. Conversion of glycogen to glucose ANSWER: E 2. The regulation of normal blood sugar level is accomplished by A. Insulin, glucagon and adrenalin B. Cell tissue absorption of glucose from the blood C. The breakdo ...
... C. Conversion of fat and/ or protein to glucose D. The anaerobic metabolism of glucose E. Conversion of glycogen to glucose ANSWER: E 2. The regulation of normal blood sugar level is accomplished by A. Insulin, glucagon and adrenalin B. Cell tissue absorption of glucose from the blood C. The breakdo ...
study-guide-solutions-biochemistry
... 1. A typical cell requires about 4000 different enzymes because thousands of different reactions occur in the cell and many of these reactions are facilitated by enzymes. Each enzyme is a large protein that has a specific three-dimensional shape. Its shape enables it to bind with one particular subs ...
... 1. A typical cell requires about 4000 different enzymes because thousands of different reactions occur in the cell and many of these reactions are facilitated by enzymes. Each enzyme is a large protein that has a specific three-dimensional shape. Its shape enables it to bind with one particular subs ...
Principles of BIOCHEMISTRY
... In addition to fueling the production of ATP (via glycolysis and the citric acid cycle), glucose is also a precursor of the ribose ( 核糖) and deoxyribose (去氧核糖) moieties of nucleotides (核苷 酸) and deoxynucleotides (去氧核苷酸) . The pentose phosphate pathway (戊糖磷酸途徑) is responsible for the synthesis of ri ...
... In addition to fueling the production of ATP (via glycolysis and the citric acid cycle), glucose is also a precursor of the ribose ( 核糖) and deoxyribose (去氧核糖) moieties of nucleotides (核苷 酸) and deoxynucleotides (去氧核苷酸) . The pentose phosphate pathway (戊糖磷酸途徑) is responsible for the synthesis of ri ...
Amino Acid Metabolism
... and leucine yielding CO2, and acyl-CoA derivatives. • Shares ancestry with pyruvate dehydrogenase complex, -KG dehydrogenase complex – another example of gene duplication ...
... and leucine yielding CO2, and acyl-CoA derivatives. • Shares ancestry with pyruvate dehydrogenase complex, -KG dehydrogenase complex – another example of gene duplication ...
Part 1 - ISpatula
... – Kingdom Plantae – Kingdom Animalia – Kingdom Fungi – Kingdom Bacteria • These processes demonstrate the fundamental unity of all living matter, and are collectively described as primary metabolism, with the compounds involved in the pathways being termed primary metabolites ...
... – Kingdom Plantae – Kingdom Animalia – Kingdom Fungi – Kingdom Bacteria • These processes demonstrate the fundamental unity of all living matter, and are collectively described as primary metabolism, with the compounds involved in the pathways being termed primary metabolites ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑