Unit 3 Goals - kimscience.com
... o complete and balance combustion reactions of organic molecules containing carbon, hydrogen and oxygen, and explain the reaction in terms of bonds breaking and forming, enthalpy, and entropy change. o distinguish between complete and incomplete combustion in terms of reaction conditions, resulting ...
... o complete and balance combustion reactions of organic molecules containing carbon, hydrogen and oxygen, and explain the reaction in terms of bonds breaking and forming, enthalpy, and entropy change. o distinguish between complete and incomplete combustion in terms of reaction conditions, resulting ...
ethane - Chemistry at Winthrop University
... The resolution of a racemic mixture cannot be accomplished using standard physical means (e. g. distillation, recrystallization, or chromatography), because enantiomers have identical physical properties, except for the direction of rotation of plane polarized light. To distinguish between the compo ...
... The resolution of a racemic mixture cannot be accomplished using standard physical means (e. g. distillation, recrystallization, or chromatography), because enantiomers have identical physical properties, except for the direction of rotation of plane polarized light. To distinguish between the compo ...
1. Rank the following compounds in order of decreasing acidity (1
... 2. Show the enol tautomer of 1,3,5-cyclohexatrione. Would you expect this compound to exist predominantly in the keto or enol form? O ...
... 2. Show the enol tautomer of 1,3,5-cyclohexatrione. Would you expect this compound to exist predominantly in the keto or enol form? O ...
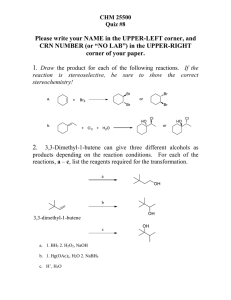
Pre Ch15 HW
... 6. Determining reactants and products in reactions of alcohols, alkyl halides, and amines (SP 15.4) 7. Determining the products in a stepwise reaction sequence (SP 15.5) 8. Determining the reactants in reactions involving aldehydes and ketones (SP 15.5) 9. Determining reactants and products in react ...
... 6. Determining reactants and products in reactions of alcohols, alkyl halides, and amines (SP 15.4) 7. Determining the products in a stepwise reaction sequence (SP 15.5) 8. Determining the reactants in reactions involving aldehydes and ketones (SP 15.5) 9. Determining reactants and products in react ...
1-Ethyl-4-hydroxy-2-oxo-1,2-dihydroquinoline-3
... 4-Hydroxy-2-quinolines. 193. Synthesis, Structure and antitubercular activity of 4hydroxy-1-isobutyl-2-oxo-1, 2, 5, 6, 7, 8-hexahydroquinoline-3-carbocylic acid N-Ramides. (Chemistry of Heterocyclic Compounds 2010). Abstract: For example of anilides and hetarylamides 4-1-isobutyl-2-oxo-1, 2, 5, 6, 7 ...
... 4-Hydroxy-2-quinolines. 193. Synthesis, Structure and antitubercular activity of 4hydroxy-1-isobutyl-2-oxo-1, 2, 5, 6, 7, 8-hexahydroquinoline-3-carbocylic acid N-Ramides. (Chemistry of Heterocyclic Compounds 2010). Abstract: For example of anilides and hetarylamides 4-1-isobutyl-2-oxo-1, 2, 5, 6, 7 ...
Organometallic Chemistry
... • The use of enantiopure allylic boranes in reactions with achiral aldehydes results not only in high diastereoselection, but also in high enantioselection. • Pure (Z)- and (E)- crotyldiisopino campheylboranes can be prepared at low temperature from (Z)- or (E)- crotylpotassium and B-methoxydiisopin ...
... • The use of enantiopure allylic boranes in reactions with achiral aldehydes results not only in high diastereoselection, but also in high enantioselection. • Pure (Z)- and (E)- crotyldiisopino campheylboranes can be prepared at low temperature from (Z)- or (E)- crotylpotassium and B-methoxydiisopin ...
Part II Biochemistry
... • Carbohydrates are now defined as: 1. polyhydroxyaldehydes, 2. polyhydroxyketones, or 3. substances that give such compounds on hydrolysis. ...
... • Carbohydrates are now defined as: 1. polyhydroxyaldehydes, 2. polyhydroxyketones, or 3. substances that give such compounds on hydrolysis. ...
III. ORGANIC CHEMISTRY Reactions
... Fractions are left in complete combustions equations, because in most cases they are written as thermochemical equations. ...
... Fractions are left in complete combustions equations, because in most cases they are written as thermochemical equations. ...
7-1 EXPERIMENT 7: Reduction of Carbonyl Compounds – Achiral
... common reducing reagents, LiAlH4 is the more powerful, reducing most carbonyl containing compounds including aldehydes, ketones, esters, and amides. A more selective reducing agent is NaBH4, which only reacts with aldehydes and ketones due to its milder nature. Both reducing agents are a source of H ...
... common reducing reagents, LiAlH4 is the more powerful, reducing most carbonyl containing compounds including aldehydes, ketones, esters, and amides. A more selective reducing agent is NaBH4, which only reacts with aldehydes and ketones due to its milder nature. Both reducing agents are a source of H ...
Chemical Reactions
... mass of the products is always equal to the mass of the reactants, is known as the law of conservation of mass ...
... mass of the products is always equal to the mass of the reactants, is known as the law of conservation of mass ...
Enantioselective Henry Reactions under Dual Lewis Acid/Amine
... selectivity (entry 1), whereas increasing the ligand loading above 45 mol % did not improve the result. The quantity of iPr2EtN was crucial too. Lower loading or absence of iPr2EtN (entries 3 and 4) led to diminished yields and ee values. Interestingly, the absence of iPr2EtN could be partially comp ...
... selectivity (entry 1), whereas increasing the ligand loading above 45 mol % did not improve the result. The quantity of iPr2EtN was crucial too. Lower loading or absence of iPr2EtN (entries 3 and 4) led to diminished yields and ee values. Interestingly, the absence of iPr2EtN could be partially comp ...
Ppt09(Wk14)Organic_final_topics
... • Enantiomers have all the same physical properties except one – the direction they rotate the plane of plane-polarized light – each one of the enantiomers will rotate the plane the same amount, but in opposite directions – that’s why enantiomers are called “optical isomers” ...
... • Enantiomers have all the same physical properties except one – the direction they rotate the plane of plane-polarized light – each one of the enantiomers will rotate the plane the same amount, but in opposite directions – that’s why enantiomers are called “optical isomers” ...
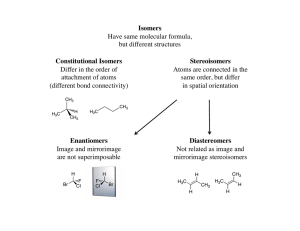
Isomers and Isomerism Isomers
... a pair of enantiomers will react different only with chiral reagents. Because living systems usually react with only one of a pair of enanatiomers, the living system is also chiral. The part of the living system that is chiral is the enzyme. An enzyme has the ability to react with only one of a pair ...
... a pair of enantiomers will react different only with chiral reagents. Because living systems usually react with only one of a pair of enanatiomers, the living system is also chiral. The part of the living system that is chiral is the enzyme. An enzyme has the ability to react with only one of a pair ...
Ppt09(Wk14)Organic_final_topics
... • Enantiomers have all the same physical properties except one – the direction they rotate the plane of plane-polarized light – each one of the enantiomers will rotate the plane the same amount, but in opposite directions – that’s why enantiomers are called “optical isomers” ...
... • Enantiomers have all the same physical properties except one – the direction they rotate the plane of plane-polarized light – each one of the enantiomers will rotate the plane the same amount, but in opposite directions – that’s why enantiomers are called “optical isomers” ...
Slides for Chapter 1-4 - Department of Chemistry and Physics
... Nucleophiles that are Brønsted bases produce elimination ...
... Nucleophiles that are Brønsted bases produce elimination ...
An Efficient Synthetic Route to Glycoamino Acid Building Blocks for
... excess of amino acid with TBTU, HOBt, and DIPEA in DMF for 4 h, followed by Fmoc deprotection using 20% piperidine in DMF for 1 h. All amino acid coupling steps were single couplings except for coupling of the glycoamino acids 4a and 4b, which were incorporated into peptide using double coupling bef ...
... excess of amino acid with TBTU, HOBt, and DIPEA in DMF for 4 h, followed by Fmoc deprotection using 20% piperidine in DMF for 1 h. All amino acid coupling steps were single couplings except for coupling of the glycoamino acids 4a and 4b, which were incorporated into peptide using double coupling bef ...
Dr Davids Essential Chemistry Definitions Bk1
... A molecule that is non-superimposable on its mirror image; such a molecule is optically active (meaning that it will rotate the plane of plane polarised light to the right or to the left). Chiral molecules frequently contain one or more asymmetric carbon atoms. Conjugate acid-base pairs: These are f ...
... A molecule that is non-superimposable on its mirror image; such a molecule is optically active (meaning that it will rotate the plane of plane polarised light to the right or to the left). Chiral molecules frequently contain one or more asymmetric carbon atoms. Conjugate acid-base pairs: These are f ...
Chapter 4 mastery check
... they have different chemical properties they have the same molecular formula their atoms and bonds are arranged in different sequences they are a result of restricted movement around a carbon double bond their possible numbers increase as carbon skeletons increase in size. ...
... they have different chemical properties they have the same molecular formula their atoms and bonds are arranged in different sequences they are a result of restricted movement around a carbon double bond their possible numbers increase as carbon skeletons increase in size. ...
14. The Direct and Enantioselective Organocatalytic -Oxidation of Aldehydes
... Supporting Information Available: Experimental procedures, structural proofs, and spectral data for all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org. References ...
... Supporting Information Available: Experimental procedures, structural proofs, and spectral data for all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org. References ...
Chapter 4
... If the organic molecules interacting with the protein are also chiral, then there will be an energy difference depending upon which enantiomer is interacting with the protein ...
... If the organic molecules interacting with the protein are also chiral, then there will be an energy difference depending upon which enantiomer is interacting with the protein ...
Job Description
... medicinal chemists (including Scientists at the University of Liverpool supervised by Prof P O’Neil) involved in the discovery and development of novel peroxide drug candidates targeting malaria. ...
... medicinal chemists (including Scientists at the University of Liverpool supervised by Prof P O’Neil) involved in the discovery and development of novel peroxide drug candidates targeting malaria. ...
Enantioselective synthesis

Enantioselective synthesis, also called chiral synthesis or asymmetric synthesis, is defined by IUPAC as: a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric (enantiomeric or diastereoisomeric) products in unequal amounts.Put more simply: it is the synthesis of a compound by a method that favors the formation of a specific enantiomer or diastereomer.Enantioselective synthesis is a key process in modern chemistry and is particularly important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity.