Document
... reaction mixture was stirred for 1 min and placed in freezer (20 °C) for 2 days without stirring. The reaction mixture was monitored by 19F NMR for conversion and diastereoselectivity, and loaded on to preparative TLC plate. In most cases, the diastereomers can be separated with hexane/ethyl acetate ...
... reaction mixture was stirred for 1 min and placed in freezer (20 °C) for 2 days without stirring. The reaction mixture was monitored by 19F NMR for conversion and diastereoselectivity, and loaded on to preparative TLC plate. In most cases, the diastereomers can be separated with hexane/ethyl acetate ...
Limitations in Determining Enantiomeric Excess of Alcohols by 31P
... Following the global trend of working with enantiomerically enriched mixtures or pure enantiomers has brought a new problem to our research group, namely, enantiomeric discrimination. Chiral GC and HPLC columns and chiral eluents are not always efficient thus other methods of analysis to assess enan ...
... Following the global trend of working with enantiomerically enriched mixtures or pure enantiomers has brought a new problem to our research group, namely, enantiomeric discrimination. Chiral GC and HPLC columns and chiral eluents are not always efficient thus other methods of analysis to assess enan ...
CBS Reduction
... History of CBS reduction and selectivity • In 1981 Itsuno et al reduced achiral ketones to chiral alcohols using alkoxy-amine-borane complexes in enantioselectivity and in high yield. • In 1987 E.J. Corey, with his co-workers, used oxazaborolidines to rapidlly reduce ketones in the presence of BH3- ...
... History of CBS reduction and selectivity • In 1981 Itsuno et al reduced achiral ketones to chiral alcohols using alkoxy-amine-borane complexes in enantioselectivity and in high yield. • In 1987 E.J. Corey, with his co-workers, used oxazaborolidines to rapidlly reduce ketones in the presence of BH3- ...
Carolina Aguirre, Rosa Arrieta, Soledad Anjarí, Andrés Illanes
... Penicillin acylase (penicillin amidohydrolase;EC 3.5.1.11) is a moderately priced readily available enzyme (1) with remarkable versatility (2). It is currently used for the industrial production of 6-aminopenicillanic acid (6-A PA ) by hydrolysis of penicillin G (3) or V (4) and 7-aminodesacetoxycep ...
... Penicillin acylase (penicillin amidohydrolase;EC 3.5.1.11) is a moderately priced readily available enzyme (1) with remarkable versatility (2). It is currently used for the industrial production of 6-aminopenicillanic acid (6-A PA ) by hydrolysis of penicillin G (3) or V (4) and 7-aminodesacetoxycep ...
Microsoft Word
... The selective oxidation of alcohols to carbonyl compounds is the foundation of many important industrial and fine-chemical processes. Many stoichiometric oxidants are known for the oxidation of alcohols such as chromates, hypochlorite, permanganates, and others, however many of these oxidants are to ...
... The selective oxidation of alcohols to carbonyl compounds is the foundation of many important industrial and fine-chemical processes. Many stoichiometric oxidants are known for the oxidation of alcohols such as chromates, hypochlorite, permanganates, and others, however many of these oxidants are to ...
Formative 3.5 2014
... (ii) Amino acids can form polymers because at each end of the molecule is a functional group that can react with a functional group from neighbouring molecules. (b) To be able to form enantiomers a molecule must have a chiral atom – one to which four different groups are attached. This enables the f ...
... (ii) Amino acids can form polymers because at each end of the molecule is a functional group that can react with a functional group from neighbouring molecules. (b) To be able to form enantiomers a molecule must have a chiral atom – one to which four different groups are attached. This enables the f ...
Information Regarding Prof
... A new way for 1,3-amino alcohols to undergo stereoselective synthesis based on the Inmediated allylation of keto ester (R,S)-I in aqueous media, resulting from shielding of one face by a remote substituent, developed to be a useful method for acyclic stereocontrol in the absence of any steric intera ...
... A new way for 1,3-amino alcohols to undergo stereoselective synthesis based on the Inmediated allylation of keto ester (R,S)-I in aqueous media, resulting from shielding of one face by a remote substituent, developed to be a useful method for acyclic stereocontrol in the absence of any steric intera ...
Biochemistry Carbon Compounds Supplement 1 Name: . Answer
... Answer the following questions on a separate sheet of paper and staple this handout and the answers together. This handout should be the top sheet. Vocabulary: Organic Compound functional group monomer hydrolysis adenosine triphosphate (ATP) ...
... Answer the following questions on a separate sheet of paper and staple this handout and the answers together. This handout should be the top sheet. Vocabulary: Organic Compound functional group monomer hydrolysis adenosine triphosphate (ATP) ...
Asymmetric Catalytic Aldol
... • Require mild conditions • Their reactions are often compatible with one another making one-pot reactions feasible • Environmentally friendly • However narrow substrate tolerance! • Two types of enzymatic catalysts that effect aldol addition: a) The aldolases: a group of naturally occurring enzymes ...
... • Require mild conditions • Their reactions are often compatible with one another making one-pot reactions feasible • Environmentally friendly • However narrow substrate tolerance! • Two types of enzymatic catalysts that effect aldol addition: a) The aldolases: a group of naturally occurring enzymes ...
Worksheet 1 - Oregon State chemistry
... Addition is to make an addition to a molecule. An example is the addition of a small molecule (such as Br2) to an alkene. ...
... Addition is to make an addition to a molecule. An example is the addition of a small molecule (such as Br2) to an alkene. ...
Pyrrolidine-2-carboxylic Acid (l
... activity, in catalyzing a wide variety of reactions such as aldol,1,2 Mannich,3–5,10–12 Michael,6–9 in a highly enantioselective manner. These reactions have produced a variety of useful chiral materials for organic synthesis. Most of the L-Proline (I) catalyzed reactions are believed to involve ena ...
... activity, in catalyzing a wide variety of reactions such as aldol,1,2 Mannich,3–5,10–12 Michael,6–9 in a highly enantioselective manner. These reactions have produced a variety of useful chiral materials for organic synthesis. Most of the L-Proline (I) catalyzed reactions are believed to involve ena ...
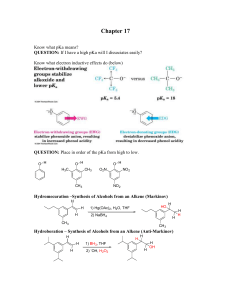
Chapter 17 - Ellis Benjamin
... Chapter 17 Know what pKa means? QUESTION: If I have a high pKa will I dissociates easily? Know what electron inductive effects do (below) ...
... Chapter 17 Know what pKa means? QUESTION: If I have a high pKa will I dissociates easily? Know what electron inductive effects do (below) ...
lect3
... 3. Grignard reagents in solution involve many complex equilibria between several organometallic species 4. Organometallic compounds of the light elements (e.g. Li, Be, Mg, Al) are often electron deficient, with bridging R groups and 2-electron 3-centre bonds. 5. These electron deficient compounds te ...
... 3. Grignard reagents in solution involve many complex equilibria between several organometallic species 4. Organometallic compounds of the light elements (e.g. Li, Be, Mg, Al) are often electron deficient, with bridging R groups and 2-electron 3-centre bonds. 5. These electron deficient compounds te ...
• Pergamon
... preparation of the corresponding 1,9-dicyanodipyrromethanes. 3 These dicyano compounds have been successfully employed in the synthesis of porphacyanins, a new class of "expanded" porphyrins4,5 which have become the focus of much research activity owing to their use in the complexation of large cati ...
... preparation of the corresponding 1,9-dicyanodipyrromethanes. 3 These dicyano compounds have been successfully employed in the synthesis of porphacyanins, a new class of "expanded" porphyrins4,5 which have become the focus of much research activity owing to their use in the complexation of large cati ...
Practice problems for week 8 PDF
... A meso compound is one that contains chiral centres but is achiral because of an intermal plane of symmetry. Thus, switching the R,S configuration does not produce a unique enantiomer but rather just another copy of the same compound. ...
... A meso compound is one that contains chiral centres but is achiral because of an intermal plane of symmetry. Thus, switching the R,S configuration does not produce a unique enantiomer but rather just another copy of the same compound. ...
131 Learning Objectives
... Draw constitutional isomers and ID longest carbon chain ID 1°, 2°, 3°, 4° carbons Name simple alkanes and cycloalkanes Predict products of combustion & balance reaction equations Chapter 13: Unsaturated Hydrocarbons Identify unsaturated hydrocarbons: alkenes, alkynes, aromatic compounds ...
... Draw constitutional isomers and ID longest carbon chain ID 1°, 2°, 3°, 4° carbons Name simple alkanes and cycloalkanes Predict products of combustion & balance reaction equations Chapter 13: Unsaturated Hydrocarbons Identify unsaturated hydrocarbons: alkenes, alkynes, aromatic compounds ...
Enantiodivergent conversion of chiral secondary alcohols into
... •Only works for aryl alcohols •Aryl boranes (e.g. Ph-9-BBN) incompatible due to protodeboronation during aqueous oxidative work-up •Indanol-derived carbamate gives same enantiomer (retention) with triethyl borane or ethylboronic acid (pyramidalization of geometrically constrained carbanion) ...
... •Only works for aryl alcohols •Aryl boranes (e.g. Ph-9-BBN) incompatible due to protodeboronation during aqueous oxidative work-up •Indanol-derived carbamate gives same enantiomer (retention) with triethyl borane or ethylboronic acid (pyramidalization of geometrically constrained carbanion) ...
Enantioselective synthesis

Enantioselective synthesis, also called chiral synthesis or asymmetric synthesis, is defined by IUPAC as: a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric (enantiomeric or diastereoisomeric) products in unequal amounts.Put more simply: it is the synthesis of a compound by a method that favors the formation of a specific enantiomer or diastereomer.Enantioselective synthesis is a key process in modern chemistry and is particularly important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity.