Today`s literature presentation = 2/5th trivia + 2/5th
... Heavy metals used in most of theses cause problems in removal, must be present <10 ppm levels. Many oxidizing agents are high energy species, giving rise to thermal hazards at scale. ...
... Heavy metals used in most of theses cause problems in removal, must be present <10 ppm levels. Many oxidizing agents are high energy species, giving rise to thermal hazards at scale. ...
print
... Oxidizing Agents with Metal Oxygen Bonds • The most common oxidizing agents with metal-oxygen bonds contain either chromium +6 or manganese +7. • Common Cr6+ reagents include CrO3 and sodium or potassium dichromate (Na2Cr2O7 and K2Cr2O7). • Pyridinium chlorochromate (PCC) is a more selective Cr6+ ...
... Oxidizing Agents with Metal Oxygen Bonds • The most common oxidizing agents with metal-oxygen bonds contain either chromium +6 or manganese +7. • Common Cr6+ reagents include CrO3 and sodium or potassium dichromate (Na2Cr2O7 and K2Cr2O7). • Pyridinium chlorochromate (PCC) is a more selective Cr6+ ...
conversion of the OH group into a better leaving group, and
... • Although entropy favors product formation in dehydration (i.e., one molecule of reactant forms two molecules of product), enthalpy does not, since the bonds broken in the reactant are stronger than the and bonds formed in the products. ...
... • Although entropy favors product formation in dehydration (i.e., one molecule of reactant forms two molecules of product), enthalpy does not, since the bonds broken in the reactant are stronger than the and bonds formed in the products. ...
Aldehydes and Ketones
... Acetals are used to “protect” the carbonyl groups of aldehydes and ketones when one wants to have some other part of the molecule react without affecting the aldehyde or ketone functional group. They can be used this way because they are fairly unreactive and the carbonyl functional group can be reg ...
... Acetals are used to “protect” the carbonyl groups of aldehydes and ketones when one wants to have some other part of the molecule react without affecting the aldehyde or ketone functional group. They can be used this way because they are fairly unreactive and the carbonyl functional group can be reg ...
Organometallics II
... formula C11H22O, are formed in the reaction of methyl lithium with 3-(R)-tertbutylcyclohexanone. These two alcohols are ...
... formula C11H22O, are formed in the reaction of methyl lithium with 3-(R)-tertbutylcyclohexanone. These two alcohols are ...
Reactions of Molecules with Oxygen
... Oxidation Reactions of Alcohols What oxidizing agent is used in the selective oxidation of alcohols? Potassium dichromate, K2Cr2O7, is the oxidizing agent [O] used in many alcohol oxidation reactions. The K2Cr2O7 solution must be acidified first by adding sulfuric acid, H2SO4. This provides an appr ...
... Oxidation Reactions of Alcohols What oxidizing agent is used in the selective oxidation of alcohols? Potassium dichromate, K2Cr2O7, is the oxidizing agent [O] used in many alcohol oxidation reactions. The K2Cr2O7 solution must be acidified first by adding sulfuric acid, H2SO4. This provides an appr ...
OxorheniumCatalyzed Deoxydehydration of Sugars and Sugar
... in examining the use of other inexpensive/bio-derived alcohols. We noted that [Re2(CO)10] and [BrRe(CO)5] catalysts employed in the original report of Bergman and Ellman[8] required air and high temperature for activation. We postulated that the actual active catalyst may be an oxidized rhenium spec ...
... in examining the use of other inexpensive/bio-derived alcohols. We noted that [Re2(CO)10] and [BrRe(CO)5] catalysts employed in the original report of Bergman and Ellman[8] required air and high temperature for activation. We postulated that the actual active catalyst may be an oxidized rhenium spec ...
Alcohols: Structure and Physical Properties
... oxygen and hydrogen, hydrogen bonds can form between alcohol molecules (Figure 1.2). As a result of this intermolecular hydrogen bonding, alcohols boil at much higher temperatures than hydrocarbons of similar molecular weight. These higher boiling points are caused by the large amount of heat needed ...
... oxygen and hydrogen, hydrogen bonds can form between alcohol molecules (Figure 1.2). As a result of this intermolecular hydrogen bonding, alcohols boil at much higher temperatures than hydrocarbons of similar molecular weight. These higher boiling points are caused by the large amount of heat needed ...
Week 11 Problem Set (Solutions)
... (without stereochem) to help you visualize which isomer(s) would be appropriate for specific boxes. Looking at the top section, let’s start by filling in with what we know. Only two structures are provided – the rest of the information is given with reagents. This tests out ability to work backwards ...
... (without stereochem) to help you visualize which isomer(s) would be appropriate for specific boxes. Looking at the top section, let’s start by filling in with what we know. Only two structures are provided – the rest of the information is given with reagents. This tests out ability to work backwards ...
alcohols!
... • big alcohols have big nonpolar parts and are less soluble in water • Small ones are more soluble b/c they are more like ...
... • big alcohols have big nonpolar parts and are less soluble in water • Small ones are more soluble b/c they are more like ...
montmorillonite catalysts for ethylene hydration
... concentration of ethanol in the product stream. Ethanol was produced with a selectivity of 95% (based on water) with diethyl ether as the major by-product. Al-montmorillonite gave the highest conversions of ethylene and water to ethanol (Fig. 4). A 5% conversion (based on water) per pass was obtaine ...
... concentration of ethanol in the product stream. Ethanol was produced with a selectivity of 95% (based on water) with diethyl ether as the major by-product. Al-montmorillonite gave the highest conversions of ethylene and water to ethanol (Fig. 4). A 5% conversion (based on water) per pass was obtaine ...
Reactions of Oxacyclopropanes
... LiAlH4 can open the rings of oxacyclopropanes to yield alcohols. (Ordinary ethers do not react.) In asymmetrical systems, the hydride attacks the less substituted side. ...
... LiAlH4 can open the rings of oxacyclopropanes to yield alcohols. (Ordinary ethers do not react.) In asymmetrical systems, the hydride attacks the less substituted side. ...
4.5: Bonding in Alcohols and Alkyl Halides
... If an electron pair moves in on a new atom, another electron pair must leave so that the atom does not exceed a full valance of eight electrons. There are two common exceptions: A. B. ...
... If an electron pair moves in on a new atom, another electron pair must leave so that the atom does not exceed a full valance of eight electrons. There are two common exceptions: A. B. ...
enantioselective zeolite-catalyzed reactions
... Kinetic Resolution The use of zeolite-catalyzed, enantioselective reactions has been expanded to kinetic resolution. The first reported case involves the use of chiral dithiane oxides adsorbed onto zeolite Y for the selective dehydration of racemic 2-butanol.20-21 Although this reaction shows signif ...
... Kinetic Resolution The use of zeolite-catalyzed, enantioselective reactions has been expanded to kinetic resolution. The first reported case involves the use of chiral dithiane oxides adsorbed onto zeolite Y for the selective dehydration of racemic 2-butanol.20-21 Although this reaction shows signif ...
Alcohols - Science Skool!
... If a small piece of sodium is dropped into some ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colourless solution of sodium ethoxide, CH3CH2ONa. Sodium ethoxide is known as an alkoxide and is very ...
... If a small piece of sodium is dropped into some ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colourless solution of sodium ethoxide, CH3CH2ONa. Sodium ethoxide is known as an alkoxide and is very ...
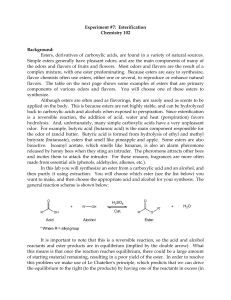
ESTERIFICATION
... this means is that once the reaction reaches equilibrium, there could be a large amount of starting material remaining, resulting in a poor yield of the ester. In order to resolve this problem we make use of Le Chatelier's principle, which predicts that we can drive the equilibrium to the right (to ...
... this means is that once the reaction reaches equilibrium, there could be a large amount of starting material remaining, resulting in a poor yield of the ester. In order to resolve this problem we make use of Le Chatelier's principle, which predicts that we can drive the equilibrium to the right (to ...
Lab 7_Esterification
... this means is that once the reaction reaches equilibrium, there could be a large amount of starting material remaining, resulting in a poor yield of the ester. In order to resolve this problem we make use of Le Chatelier's principle, which predicts that we can drive the equilibrium to the right (to ...
... this means is that once the reaction reaches equilibrium, there could be a large amount of starting material remaining, resulting in a poor yield of the ester. In order to resolve this problem we make use of Le Chatelier's principle, which predicts that we can drive the equilibrium to the right (to ...
Highlights IACChE`s James Y. Oldshue Lecture Tuesday, November
... requires a detailed knowledge on the relationship between the chemical nature of the solvents and the interactions taking place in the gas-liquid-solid catalytic systems. One of the most common types of catalytic reactions carried out in the presence of solvents is the hydrogenation of organic comp ...
... requires a detailed knowledge on the relationship between the chemical nature of the solvents and the interactions taking place in the gas-liquid-solid catalytic systems. One of the most common types of catalytic reactions carried out in the presence of solvents is the hydrogenation of organic comp ...
Gas Chromatography: Analyzing Alkene Isomers David L. Flanigan
... using a dilute solution of sulfuric acid. The reaction resulted in a mixture of alkenes that was purified by distillation and analyzed using gas chromatography. Theoretical Background: Alcohols can be dehydrated using catalytic acid conditions to give alkenes. H+ ...
... using a dilute solution of sulfuric acid. The reaction resulted in a mixture of alkenes that was purified by distillation and analyzed using gas chromatography. Theoretical Background: Alcohols can be dehydrated using catalytic acid conditions to give alkenes. H+ ...
Chapter 11: Alcohols and Ethers
... • Alcohol Groups do not “Survive” Many Organic Reactions • Alkylation (Ether Formation) Protects OH’s During Synthesis • Can Remove the Protecting Group w/ Dilute Aqueous Acid • Generally Dissolve Alcohol in Acid, THEN add Isobutylene • Addition in this Manner Minimizes Isobutylene Dimerization • Le ...
... • Alcohol Groups do not “Survive” Many Organic Reactions • Alkylation (Ether Formation) Protects OH’s During Synthesis • Can Remove the Protecting Group w/ Dilute Aqueous Acid • Generally Dissolve Alcohol in Acid, THEN add Isobutylene • Addition in this Manner Minimizes Isobutylene Dimerization • Le ...
Document
... • Green chemistry is the use of environmentally benign methods to synthesize compounds. • Its purpose is to use safer reagents and less solvent, and develop reactions that form fewer by-products and generate less waste. • Since many oxidation methods use toxic reagents (such as OsO4 and O3) and corr ...
... • Green chemistry is the use of environmentally benign methods to synthesize compounds. • Its purpose is to use safer reagents and less solvent, and develop reactions that form fewer by-products and generate less waste. • Since many oxidation methods use toxic reagents (such as OsO4 and O3) and corr ...
Chapter 7 Alkenes and Alkynes I
... Primary alcohols cannot undergo E1 dehydration because of the instability of the carbocation-like transition state in the 2nd step In the E2 dehydration the first step is again protonation of the hydroxyl to yield the good leaving group water ...
... Primary alcohols cannot undergo E1 dehydration because of the instability of the carbocation-like transition state in the 2nd step In the E2 dehydration the first step is again protonation of the hydroxyl to yield the good leaving group water ...
Carboxylic acids from primary alcohols and aldehydes by a
... NMR spectra of the products showed only the desired products and no unreacted starting material was observed. ...
... NMR spectra of the products showed only the desired products and no unreacted starting material was observed. ...
Application of IBX Method for the Synthesis of Ketones from
... One of the most common reactions in chemistry is the synthesis of ketones from acids1−4 . First, the acid is changed into its chloride. Then the acid chloride reacts with an organometallic reagent or gives a FriedelCrafts type reaction in the presence of Lewis acids. These methods are very useful fo ...
... One of the most common reactions in chemistry is the synthesis of ketones from acids1−4 . First, the acid is changed into its chloride. Then the acid chloride reacts with an organometallic reagent or gives a FriedelCrafts type reaction in the presence of Lewis acids. These methods are very useful fo ...
PowerPoint 프레젠테이션
... 1928–, b. Methuen, Mass., grad. Massachusetts Institute of Technology (B.S. 1948, Ph.D. 1951). In 1990, he was awarded the Nobel Prize in Chemistry. ...
... 1928–, b. Methuen, Mass., grad. Massachusetts Institute of Technology (B.S. 1948, Ph.D. 1951). In 1990, he was awarded the Nobel Prize in Chemistry. ...
Kinetic resolution

In organic chemistry, kinetic resolution is a means of differentiating two enantiomers in a racemic mixture. In kinetic resolution, two enantiomers react with different reaction rates in a chemical reaction with a chiral catalyst or reagent, resulting in an enantioenriched sample of the less reactive enantiomer. As opposed to chiral resolution, kinetic resolution does not rely on different physical properties of diastereomeric products, but rather on the different chemical properties of the racemic starting materials. This enantiomeric excess (ee) of the unreacted starting material continually rises as more product is formed, reaching 100% just before full completion of the reaction. Kinetic resolution relies upon differences in reactivity between enantiomers or enantiomeric complexes. Kinetic resolution is a concept in organic chemistry and can be used for the preparation of chiral molecules in organic synthesis. Kinetic resolution reactions utilizing purely synthetic reagents and catalysts are much less common than the use of enzymatic kinetic resolution in application towards organic synthesis, although a number of useful synthetic techniques have been developed in the past 30 years.