Facile Oxidation of Benzyl Alcohols with Sodium Nitrate/p
... yields of benzaldehydes. Although there have been reported a couple of solid supported transition metal nitrate oxidants, to the best of our knowledge, the present protocol represents the first example of the successful application of an alkali metal nitrate toward oxidation of benzyl alcohols. Trea ...
... yields of benzaldehydes. Although there have been reported a couple of solid supported transition metal nitrate oxidants, to the best of our knowledge, the present protocol represents the first example of the successful application of an alkali metal nitrate toward oxidation of benzyl alcohols. Trea ...
Hydrocarbon Derivatives:
... • dipole-dipole: intermediate strength – molecule has atoms with different ...
... • dipole-dipole: intermediate strength – molecule has atoms with different ...
heptane
... phantom(a))&&(phantom(a))&& &(H phantom(W))&&&C&(H_3)end(array)... Part A The name of the molecule shown is ...
... phantom(a))&&(phantom(a))&& &(H phantom(W))&&&C&(H_3)end(array)... Part A The name of the molecule shown is ...
- Iranian Chemical Communication
... trihalides [11] and CsF–Celite have also been utilized to achieve the acylated products of alcohols, phenols and thiols[12-13]. However, most of these protocols have some limitations such as: use of very strong basic catalyst, low yields, high temperature and longer reaction time [5,8,13,14]. But, i ...
... trihalides [11] and CsF–Celite have also been utilized to achieve the acylated products of alcohols, phenols and thiols[12-13]. However, most of these protocols have some limitations such as: use of very strong basic catalyst, low yields, high temperature and longer reaction time [5,8,13,14]. But, i ...
Kinetics of Oxidation of Aliphatic Alcohols by Potassium Dichromate
... Oxidation of alcohols has been studied extensively using different oxidizing agents and in various media.1–7 One of the most commonly used oxidants is dichromate and its derivatives. In going through the literature, one finds controversial results regarding the kinetics of these reactions though all ...
... Oxidation of alcohols has been studied extensively using different oxidizing agents and in various media.1–7 One of the most commonly used oxidants is dichromate and its derivatives. In going through the literature, one finds controversial results regarding the kinetics of these reactions though all ...
Chapter 10 for 302
... Lithium replaces the halogen This is one time where an sp3-hybridized carbon acts the same as an sp2hybridized carbon. This means that this works on any carbon-halogen bond Solvent There cannot be any acidic protons in the solvent, as the Grignard is such a strong base. There cannot be a ...
... Lithium replaces the halogen This is one time where an sp3-hybridized carbon acts the same as an sp2hybridized carbon. This means that this works on any carbon-halogen bond Solvent There cannot be any acidic protons in the solvent, as the Grignard is such a strong base. There cannot be a ...
Chapter 3. The Concept of Protecting Functional Groups
... • The protecting group reagent must react selectively (kinetic chemoselectivity) in good yield to give a protected substrate that is stable to the projected reactions. • The protecting group must be selectively removed in good yield by readily available reagents. • The protecting group should not ha ...
... • The protecting group reagent must react selectively (kinetic chemoselectivity) in good yield to give a protected substrate that is stable to the projected reactions. • The protecting group must be selectively removed in good yield by readily available reagents. • The protecting group should not ha ...
Document
... • Although entropy favors product formation in dehydration (i.e., one molecule of reactant forms two molecules of product), enthalpy does not, since the bonds broken in the reactant are stronger than the and bonds formed in the products. ...
... • Although entropy favors product formation in dehydration (i.e., one molecule of reactant forms two molecules of product), enthalpy does not, since the bonds broken in the reactant are stronger than the and bonds formed in the products. ...
Peer-reviewed Article PDF
... Dendrimers are produced in an iterative sequence of reaction steps, in which each additional interaction leads to a higher generation material. The first example of an iterative synthetic procedure toward well-defined branched structures has been reported by Vogtle, [1] who named this procedure a “c ...
... Dendrimers are produced in an iterative sequence of reaction steps, in which each additional interaction leads to a higher generation material. The first example of an iterative synthetic procedure toward well-defined branched structures has been reported by Vogtle, [1] who named this procedure a “c ...
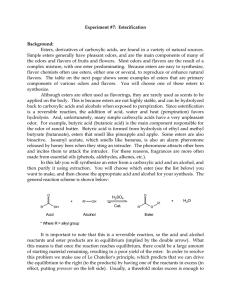
CH 102 Laboratory 7 Ester Synthesis and Smells
... low boiling point and can be rapidly removed by evaporation leaving the ester behind. Naturally this procedure will not work if the ester also has a low boiling point. Since esters can be formed from a wide variety of acids and alcohols it is not surprising that there is a very large number of possi ...
... low boiling point and can be rapidly removed by evaporation leaving the ester behind. Naturally this procedure will not work if the ester also has a low boiling point. Since esters can be formed from a wide variety of acids and alcohols it is not surprising that there is a very large number of possi ...
Ch. 14 Alcohols, Ethers, & Thiols
... Physical Properties of Alcohols • Because of increase london forces between larger molecules, the B.P. of all types of compounds, including alcohols, increase as molecular weight increases • Alcohols are much more soluble in H2O due to their H-bonding capacity. • As MW increases, the water solubili ...
... Physical Properties of Alcohols • Because of increase london forces between larger molecules, the B.P. of all types of compounds, including alcohols, increase as molecular weight increases • Alcohols are much more soluble in H2O due to their H-bonding capacity. • As MW increases, the water solubili ...
102 Lab 7 Esters Fall05
... this means is that once the reaction reaches equilibrium, there could be a large amount of starting material remaining, resulting in a poor yield of the ester. In order to resolve this problem we make use of Le Chatelier's principle, which predicts that we can drive the equilibrium to the right (to ...
... this means is that once the reaction reaches equilibrium, there could be a large amount of starting material remaining, resulting in a poor yield of the ester. In order to resolve this problem we make use of Le Chatelier's principle, which predicts that we can drive the equilibrium to the right (to ...
Organic Synthesis Part 2
... pair which combines to give an alkoxytrihydroaluminate. This can go on to donate the remaining three hydrides in the same way, although at a reduced rate. We can exploit this by deliberately making trialkoxyaluminiumhydrides, which are mild and selective reducing agents. b) sodium borohydrides the p ...
... pair which combines to give an alkoxytrihydroaluminate. This can go on to donate the remaining three hydrides in the same way, although at a reduced rate. We can exploit this by deliberately making trialkoxyaluminiumhydrides, which are mild and selective reducing agents. b) sodium borohydrides the p ...
CaCl2.2H2O assisted oxidation of alcohols with (NH4)2Cr2O7
... and the corresponding carbonyl compounds were isolated in good to high yields. No traces of carboxylic acids or other by-products were detected in all the cases studied. The only isolated products were the expected carbonyl compounds and, in the cases where the reaction had not gone to completion, t ...
... and the corresponding carbonyl compounds were isolated in good to high yields. No traces of carboxylic acids or other by-products were detected in all the cases studied. The only isolated products were the expected carbonyl compounds and, in the cases where the reaction had not gone to completion, t ...
Alcohols and Phenols - faculty at Chemeketa
... Alcohols are weak Brønsted bases Protonated by strong acids to yield oxonium ions, ...
... Alcohols are weak Brønsted bases Protonated by strong acids to yield oxonium ions, ...
File - Rasapalli Research Group
... Can be accomplished by inorganic reagents, such as KMnO4, CrO3, and Na2Cr2O7 or by more selective, expensive reagents ...
... Can be accomplished by inorganic reagents, such as KMnO4, CrO3, and Na2Cr2O7 or by more selective, expensive reagents ...
Alcohols, phenols, thiols and ethers notes
... Classify each as an alcohol (1), phenol (2), or an ether (3): A. _____ CH3CH2-O-CH3 OH ...
... Classify each as an alcohol (1), phenol (2), or an ether (3): A. _____ CH3CH2-O-CH3 OH ...
102 Lecture Ch14a
... • Find longest chain containing the C to which the OH group is attached • Number C’s starting at end nearest OH group • Locate and number substituents and give full name - use a number to indicate position of OH group - cyclic alcohols have cyclo- before the parent name; numbering begins at the OH g ...
... • Find longest chain containing the C to which the OH group is attached • Number C’s starting at end nearest OH group • Locate and number substituents and give full name - use a number to indicate position of OH group - cyclic alcohols have cyclo- before the parent name; numbering begins at the OH g ...
PDF document
... used extensively for the preparation of a variety of fine or special chemicals such as polymers, pharmaceuticals, solvents, and food additives,1 and versatile methods for the preparation of these type of compounds have been reported. However, direct conversion of primary alcohols to the correspondin ...
... used extensively for the preparation of a variety of fine or special chemicals such as polymers, pharmaceuticals, solvents, and food additives,1 and versatile methods for the preparation of these type of compounds have been reported. However, direct conversion of primary alcohols to the correspondin ...
Reaction of orthoesters with alcohols in the presence of acidic
... alcohols. However, when SiO2 is used as the catalyst, we could isolate corresponding O-acetylated compound 3 as major product (in case of primary alcohols) together with minor quantity of dimeric ethers 2 and unsymmetrical ethers 1 and this catalyst was effective in case of primary saturated (nonben ...
... alcohols. However, when SiO2 is used as the catalyst, we could isolate corresponding O-acetylated compound 3 as major product (in case of primary alcohols) together with minor quantity of dimeric ethers 2 and unsymmetrical ethers 1 and this catalyst was effective in case of primary saturated (nonben ...
Chapter 12 Alcohols from Carbonyl Compounds: Oxidation
... • Ingested ethanol is oxidized in the liver first to CH3CHO (acetaldehyde), and then to CH3COO¯ (the acetate anion). • This oxidation is catalyzed by alcohol dehydrogenase. • If more ethanol is ingested than can be metabolized, the concentration of acetaldehyde increases. Acetaldehyde, which is toxi ...
... • Ingested ethanol is oxidized in the liver first to CH3CHO (acetaldehyde), and then to CH3COO¯ (the acetate anion). • This oxidation is catalyzed by alcohol dehydrogenase. • If more ethanol is ingested than can be metabolized, the concentration of acetaldehyde increases. Acetaldehyde, which is toxi ...
THE GENERAL LAW OF CHEMICAL KINETICS, DOES IT EXIST?
... through a hole in the floor into the bell-ringer room. But let us imagine that every rope instead of putting into motion one bell participates in the motion of many parts of the mechanism and that the motion of every bell is determined not only by the motions of its own rope but the motions of sever ...
... through a hole in the floor into the bell-ringer room. But let us imagine that every rope instead of putting into motion one bell participates in the motion of many parts of the mechanism and that the motion of every bell is determined not only by the motions of its own rope but the motions of sever ...
- M E S KVM College Valanchery.
... magnesium bromide followed by a regular acidic work-up ? (a) CH3CH2CHO (c) CH3COCH3 (propanone) (e) C6H5CO2CH3 (g) CH3CH2OH ...
... magnesium bromide followed by a regular acidic work-up ? (a) CH3CH2CHO (c) CH3COCH3 (propanone) (e) C6H5CO2CH3 (g) CH3CH2OH ...
Kinetic resolution

In organic chemistry, kinetic resolution is a means of differentiating two enantiomers in a racemic mixture. In kinetic resolution, two enantiomers react with different reaction rates in a chemical reaction with a chiral catalyst or reagent, resulting in an enantioenriched sample of the less reactive enantiomer. As opposed to chiral resolution, kinetic resolution does not rely on different physical properties of diastereomeric products, but rather on the different chemical properties of the racemic starting materials. This enantiomeric excess (ee) of the unreacted starting material continually rises as more product is formed, reaching 100% just before full completion of the reaction. Kinetic resolution relies upon differences in reactivity between enantiomers or enantiomeric complexes. Kinetic resolution is a concept in organic chemistry and can be used for the preparation of chiral molecules in organic synthesis. Kinetic resolution reactions utilizing purely synthetic reagents and catalysts are much less common than the use of enzymatic kinetic resolution in application towards organic synthesis, although a number of useful synthetic techniques have been developed in the past 30 years.