carbohydrates: monosaccharides. oligo
... The formation of the cyclic form is accompanied by formation of new hydroxyl group which is absent in the open-chain form. It is called hemiacetal hydroxyl group. Its properties differ from the other hydroxyl groups because of two reasons. First of all, it is in equilibrium with the carbonyl group a ...
... The formation of the cyclic form is accompanied by formation of new hydroxyl group which is absent in the open-chain form. It is called hemiacetal hydroxyl group. Its properties differ from the other hydroxyl groups because of two reasons. First of all, it is in equilibrium with the carbonyl group a ...
Process for polymerizing olefins
... It is noted that after the ?rst hour, polymer yields, based on the ole?n, were well above 90% at the high BFazwater 45 mol ratio used, and that a substantial amount of the poly ...
... It is noted that after the ?rst hour, polymer yields, based on the ole?n, were well above 90% at the high BFazwater 45 mol ratio used, and that a substantial amount of the poly ...
CHEM120 - ORGANIC CHEMISTRY WORKSHEET 1
... Be able to determine which of two groups is the better base and be able to predict whether a substitution reaction will occur or not. If a reaction can occur, you must be able to predict the product. ...
... Be able to determine which of two groups is the better base and be able to predict whether a substitution reaction will occur or not. If a reaction can occur, you must be able to predict the product. ...
Carbonyl Compounds - Thomas Tallis Science
... dipole–dipole interactions. In addition, these molecules can form hydrogen bonds with each other, due to the slightly positive hydrogen atom of the hydroxyl group. 12 of 38 ...
... dipole–dipole interactions. In addition, these molecules can form hydrogen bonds with each other, due to the slightly positive hydrogen atom of the hydroxyl group. 12 of 38 ...
Document
... Ozonolysis of Alkenes - oxidative cleavage of an alkene to carbonyl compounds (aldehydes and ketones). The and -bonds of the alkene are broken and replaced with C=O double bonds. C=C of aryl rings, CN and C=O do not react with ozone, CC react very slowly with ozone ...
... Ozonolysis of Alkenes - oxidative cleavage of an alkene to carbonyl compounds (aldehydes and ketones). The and -bonds of the alkene are broken and replaced with C=O double bonds. C=C of aryl rings, CN and C=O do not react with ozone, CC react very slowly with ozone ...
reactions of alcohols with alkenes over an aluminum
... Figure 1. Mechanism for the acid-catalyzed reaction of 2-methyl pent-2-ene with alcohols (Al-montmorillonite catalyst). methyl t-butyl ether when using a clay catalyst (Bylina et al.. 1980: Adams et al.. 1981b). At this temperature methanol is the only alcohol to form a di-alkyl ether. whereas Balla ...
... Figure 1. Mechanism for the acid-catalyzed reaction of 2-methyl pent-2-ene with alcohols (Al-montmorillonite catalyst). methyl t-butyl ether when using a clay catalyst (Bylina et al.. 1980: Adams et al.. 1981b). At this temperature methanol is the only alcohol to form a di-alkyl ether. whereas Balla ...
Carbonyls
... Tautomers are isomers which differ in the placement of an atom of hydrogen and a double bond. The keto form has a C=O while the enol form has a C=C. The keto form is usually the most stable. ...
... Tautomers are isomers which differ in the placement of an atom of hydrogen and a double bond. The keto form has a C=O while the enol form has a C=C. The keto form is usually the most stable. ...
Practice Problem - HCC Southeast Commons
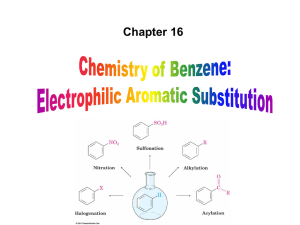
... resonance effects that reinforce each other – The ortho and para intermediates are destabilized – The positive charge of the carbocation intermediate in ortho and para attack is directly on the carbon that bears the deactivating group and resonance ...
... resonance effects that reinforce each other – The ortho and para intermediates are destabilized – The positive charge of the carbocation intermediate in ortho and para attack is directly on the carbon that bears the deactivating group and resonance ...
Ch 19 Aldehydes and Ketones
... - This results in an enolate intermediate (Nu-C-C=C-O-1) rather than an alkoxide. - This happens because the enolate is stabilized by resonance: C=C-O-1 ↔ -1C-C=O - Adding H3O+ will then protonate the anionic C, to create a saturated carbonyl product. - The carbonyl will remain, but there will be ...
... - This results in an enolate intermediate (Nu-C-C=C-O-1) rather than an alkoxide. - This happens because the enolate is stabilized by resonance: C=C-O-1 ↔ -1C-C=O - Adding H3O+ will then protonate the anionic C, to create a saturated carbonyl product. - The carbonyl will remain, but there will be ...
Pincer Complexes. Applications in Catalysis
... catalysts (1, 2) Milstein achieved full conversion in the couplings of iodobenzene with methylacrylate, using Nmethylpyrrolidine (NMP) as solvent and sodium carbonate as base with a maximum of 500,000 turnover numbers for iodobenzene and up to 132,900 for bromobencene. By the same time, Beller and Z ...
... catalysts (1, 2) Milstein achieved full conversion in the couplings of iodobenzene with methylacrylate, using Nmethylpyrrolidine (NMP) as solvent and sodium carbonate as base with a maximum of 500,000 turnover numbers for iodobenzene and up to 132,900 for bromobencene. By the same time, Beller and Z ...
enzymatic And Limited Industrial Use
... A reaction that can proceed in more than one way to produce different products involving different carbon atoms, where one predominates. It is said to be regioselective. ...
... A reaction that can proceed in more than one way to produce different products involving different carbon atoms, where one predominates. It is said to be regioselective. ...
Organic - UCLA Chemistry and Biochemistry
... Sharpless epoxidation technology and then its brominative cyclization to produce specifically the required doubly anti dihalo ether 5a which would then be easily converted into aplysiapyranoid D, Id. There was the serious question of whether one would obtain mainly the bromotetrahydropyrans or the b ...
... Sharpless epoxidation technology and then its brominative cyclization to produce specifically the required doubly anti dihalo ether 5a which would then be easily converted into aplysiapyranoid D, Id. There was the serious question of whether one would obtain mainly the bromotetrahydropyrans or the b ...
CBSEGuess.com
... reacted With HBr to give (c) which is an isomer of (a). when (a) is reacted with Na metal it give (d), C818 which is different from the compound formed when n-butyl bromide is reacted with Na metal . Give the structural formula of (a) and write the equations. ...
... reacted With HBr to give (c) which is an isomer of (a). when (a) is reacted with Na metal it give (d), C818 which is different from the compound formed when n-butyl bromide is reacted with Na metal . Give the structural formula of (a) and write the equations. ...
Chapter 24. Amines
... acceptors except in very strong acid • The C=O group is strongly electron-withdrawing, making the N a very weak base • Addition of a proton occurs on O but this destroys the double bond character of C=O as a requirement of stabilization by N ...
... acceptors except in very strong acid • The C=O group is strongly electron-withdrawing, making the N a very weak base • Addition of a proton occurs on O but this destroys the double bond character of C=O as a requirement of stabilization by N ...
Reactions of 2, 6-cycloheptadienone and 2, 7
... 1965, Garbisch4 described the preparation of 2,6cycloheptadienone (1), 2,7-~yclooctadienone (2), and other cycloallcadienones from their corresponding cycloallcanones. Although four steps are required in Garbisch's synthesis, they are described thoroughly and the overall yields are good. The reasona ...
... 1965, Garbisch4 described the preparation of 2,6cycloheptadienone (1), 2,7-~yclooctadienone (2), and other cycloallcadienones from their corresponding cycloallcanones. Although four steps are required in Garbisch's synthesis, they are described thoroughly and the overall yields are good. The reasona ...
Elimination Reactions
... The substrates that favour E1 reactions are the same that favour SN1 reactions: •A substrate bearing a good leaving group attached to a tetrahedral carbon atom. •A substrate that can form a relatively stable carbocation. The difference between E1 and SN1 reactions is in the type species which reacts ...
... The substrates that favour E1 reactions are the same that favour SN1 reactions: •A substrate bearing a good leaving group attached to a tetrahedral carbon atom. •A substrate that can form a relatively stable carbocation. The difference between E1 and SN1 reactions is in the type species which reacts ...
Elimination Reactions
... The substrates that favour E1 reactions are the same that favour SN1 reactions: •A substrate bearing a good leaving group attached to a tetrahedral carbon atom. •A substrate that can form a relatively stable carbocation. The difference between E1 and SN1 reactions is in the type species which reacts ...
... The substrates that favour E1 reactions are the same that favour SN1 reactions: •A substrate bearing a good leaving group attached to a tetrahedral carbon atom. •A substrate that can form a relatively stable carbocation. The difference between E1 and SN1 reactions is in the type species which reacts ...
Chapter 24. Amines
... acceptors except in very strong acid • The C=O group is strongly electron-withdrawing, making the N a very weak base • Addition of a proton occurs on O but this destroys the double bond character of C=O as a requirement of stabilization by N ...
... acceptors except in very strong acid • The C=O group is strongly electron-withdrawing, making the N a very weak base • Addition of a proton occurs on O but this destroys the double bond character of C=O as a requirement of stabilization by N ...
Chapter 9
... • Epoxides bonded to a chain of carbon atoms can also be named as derivatives of oxirane, the simplest epoxide having two carbons and one oxygen atom in a ring. • The oxirane ring is numbered to put the O atom at position one, and the first substituent at position two. • No number is used for a subs ...
... • Epoxides bonded to a chain of carbon atoms can also be named as derivatives of oxirane, the simplest epoxide having two carbons and one oxygen atom in a ring. • The oxirane ring is numbered to put the O atom at position one, and the first substituent at position two. • No number is used for a subs ...
biodiesel production via acid catalysis
... investigate the effect of the molar ratio of alcohol, the reaction temperature, the catalyst amount, the reaction time, and the presence of water and free fatty acids on the completeness of acid-catalyzed transesterification. Transesterification is the chemical process of converting one ester, in th ...
... investigate the effect of the molar ratio of alcohol, the reaction temperature, the catalyst amount, the reaction time, and the presence of water and free fatty acids on the completeness of acid-catalyzed transesterification. Transesterification is the chemical process of converting one ester, in th ...
Tin-Catalyzed Esterification and Transesterification Reactions: A
... Lewis acids are an important class of acid catalysts. They are milder than Brønsted acids, but their utilization has been significantly increased. Lewis acids are species with deficiency of electrons that can act activating substrates rich in electrons. Frequently, Lewis acid-base adducts are the ke ...
... Lewis acids are an important class of acid catalysts. They are milder than Brønsted acids, but their utilization has been significantly increased. Lewis acids are species with deficiency of electrons that can act activating substrates rich in electrons. Frequently, Lewis acid-base adducts are the ke ...
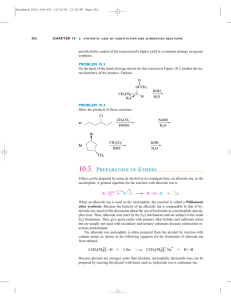
10.3 PREPARATION OF ETHERS
... However, this reaction is much less useful when ammonia is the nucleophile because the initial product, a primary amine, is a stronger base and a stronger nucleophile than is ammonia. Therefore, the primary amine preferentially reacts as the nucleophile, producing a secondary amine as a by-product ( ...
... However, this reaction is much less useful when ammonia is the nucleophile because the initial product, a primary amine, is a stronger base and a stronger nucleophile than is ammonia. Therefore, the primary amine preferentially reacts as the nucleophile, producing a secondary amine as a by-product ( ...
Manganese-Catalyzed Carbonylation of Alkyl Iodides
... in Partial Fulfillment for the Degree of Science Masters in Organic Chemistry ...
... in Partial Fulfillment for the Degree of Science Masters in Organic Chemistry ...
Baylis–Hillman reaction

The Baylis–Hillman reaction is a carbon-carbon bond forming reaction between the α-position of an activated alkene and an aldehyde, or generally a carbon electrophile. Employing a nucleophilic catalyst, such as tertiary amine and phosphine, this reaction provides a densely functionalized product (e.g. functionalized allyl alcohol in the case of aldehyde as the electrophile). This reaction is also known as the Morita–Baylis–Hillman reaction or MBH reaction. It is named for the Japanese chemist Ken-ichi Morita, the British chemist Anthony B. Baylis and the German chemist Melville E. D. Hillman.DABCO is one of the most frequently used tertiary amine catalysts for this reaction. In addition, nucleophilic amines such as DMAP and DBU as well as phosphines have been found to successfully catalyze this reaction.MBH reaction has several advantages as a useful synthetic method: 1) It is an atom-economic coupling of easily prepared starting materials. 2) Reaction of a pro-chiral electrophile generates a chiral center, therefore an asymmetric synthesis is possible. 3) Reaction products usually contain multiple functionalities in a proximity so that a variety of further transformations are possible. 4) It can employ a nucleophilic organo-catalytic system without the use of heavy metal under mild conditions.Several reviews have been written.