Quantum physics
... • Thereby Intensity ∝ Rate of incidence of photons, N/t {for a given λ} • Photocurrent I = (n/t)e, where (n/t) = rate of emission of electrons • Why rate of emission of electrons << rate of incidence of photons {for f>f0}: • Not every photon would collide with an electron; most are reflected by the ...
... • Thereby Intensity ∝ Rate of incidence of photons, N/t {for a given λ} • Photocurrent I = (n/t)e, where (n/t) = rate of emission of electrons • Why rate of emission of electrons << rate of incidence of photons {for f>f0}: • Not every photon would collide with an electron; most are reflected by the ...
Chapter
... 1. Elements in any one group have the same number of electrons in their outermost shell 2. The similarity in chemical properties among elements of the same group occurs because they have the same numbers of valence electrons 3. The number of electrons in the valence shell of an atom determines ...
... 1. Elements in any one group have the same number of electrons in their outermost shell 2. The similarity in chemical properties among elements of the same group occurs because they have the same numbers of valence electrons 3. The number of electrons in the valence shell of an atom determines ...
Chapter 10 - Lecture 3
... Structures of many-electron atoms • Because of electron correlation, no simple analytical expression for orbitals is possible • Therefore ψ(r1, r2, ….) can be expressed as ψ(r1)ψ(r2)… • Called the orbital approximation • Individual hydrogenic orbitals modified by presence of other electrons ...
... Structures of many-electron atoms • Because of electron correlation, no simple analytical expression for orbitals is possible • Therefore ψ(r1, r2, ….) can be expressed as ψ(r1)ψ(r2)… • Called the orbital approximation • Individual hydrogenic orbitals modified by presence of other electrons ...
Quantum Numbers and Periodic Table Test Review 1) Identify which
... Where c is the speed of light: 3.0 x108 m/sec Frequency and energy can be related by the formula: E = hv where h is Planck’s constant 6.63 x10-34 B. ELECTRON CONFIGURATION - address of each electron in the atom Electrons live in atom houses called orbitals. Orbitals have a distinct size shape and or ...
... Where c is the speed of light: 3.0 x108 m/sec Frequency and energy can be related by the formula: E = hv where h is Planck’s constant 6.63 x10-34 B. ELECTRON CONFIGURATION - address of each electron in the atom Electrons live in atom houses called orbitals. Orbitals have a distinct size shape and or ...
The chemical elements are fundamental building materials of matter
... • 1.B: The atoms of each element have unique structures arising from interactions between electrons and nuclei. • 1.C: Elements display periodicity in their properties when the elements are organized according to increasing atomic number. This periodicity can be explained by the regular variations t ...
... • 1.B: The atoms of each element have unique structures arising from interactions between electrons and nuclei. • 1.C: Elements display periodicity in their properties when the elements are organized according to increasing atomic number. This periodicity can be explained by the regular variations t ...
CH160: Professor Peter Sadler Introduction to inorganic chemistry
... Hence talk of probability of finding electron at point in space: determined from ψ2 (square of wave function) Atomic line spectra When atoms are excited (given energy, e.g. by heat or electrical discharge) they emit light. The light emitted has specific wavelengths (not all wavelengths). This sugges ...
... Hence talk of probability of finding electron at point in space: determined from ψ2 (square of wave function) Atomic line spectra When atoms are excited (given energy, e.g. by heat or electrical discharge) they emit light. The light emitted has specific wavelengths (not all wavelengths). This sugges ...
Energy Spectra for Fractional Quantum Hall
... Fractional quantum Hall states (FQHS) with the filling factor ν = p/q of q < 21 are examined and their energies are calculated. The classical Coulomb energy is evaluated among many electrons; that energy is linearly dependent on 1/ν. The residual binding energies are also evaluated. The electron pai ...
... Fractional quantum Hall states (FQHS) with the filling factor ν = p/q of q < 21 are examined and their energies are calculated. The classical Coulomb energy is evaluated among many electrons; that energy is linearly dependent on 1/ν. The residual binding energies are also evaluated. The electron pai ...
Electron Structure of Atoms Notes
... Ex: n = 3 is the third shell One or more orbitals with the same set of n and ℓ values are in the same sub shell Ex: n = 3 ℓ= 2 3d sub shell n=3ℓ=1 ...
... Ex: n = 3 is the third shell One or more orbitals with the same set of n and ℓ values are in the same sub shell Ex: n = 3 ℓ= 2 3d sub shell n=3ℓ=1 ...
4/10/2006 Chapter 37 Lasers, a Model Atom and Zero Point Energy
... the same wavelength as the incoming photon, b) its direction is the same as the incoming photon, c) it is in phase with the incoming photon. Thus, it is coherent. This is the mechanism for creating laser light. ...
... the same wavelength as the incoming photon, b) its direction is the same as the incoming photon, c) it is in phase with the incoming photon. Thus, it is coherent. This is the mechanism for creating laser light. ...
Lect 23 Presentation
... • Don’t have definite electron position, only a probability function. • Orbitals can have 0 angular momentum! • Each electron state labeled by 4 numbers: n = principal quantum number (1, 2, 3, …) l = angular momentum (0, 1, 2, … n-1) ml = component of l (-l < ml < l) Quantum ms = spin (-½ , +½) Numb ...
... • Don’t have definite electron position, only a probability function. • Orbitals can have 0 angular momentum! • Each electron state labeled by 4 numbers: n = principal quantum number (1, 2, 3, …) l = angular momentum (0, 1, 2, … n-1) ml = component of l (-l < ml < l) Quantum ms = spin (-½ , +½) Numb ...
1s 2s 2p - Solon City Schools
... 1. There are no values on the y axis in the tables above. Using the Periodic Table and Table 1, put numbers on the y axis. 2. Label each peak on the graphs above with s, p, d, or f to indicate the suborbital they represent.. 3. What is the total number of electrons in a neutral potassium atom? ...
... 1. There are no values on the y axis in the tables above. Using the Periodic Table and Table 1, put numbers on the y axis. 2. Label each peak on the graphs above with s, p, d, or f to indicate the suborbital they represent.. 3. What is the total number of electrons in a neutral potassium atom? ...
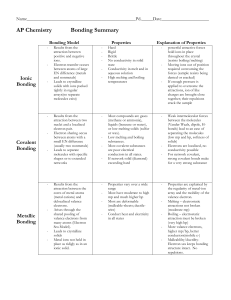
Types of Bonding Summary
... delocalized valence electrons. Arises through the shared pooling of valence electrons from many atoms (Electron Sea Model). Leads to crystalline solids Metal ions not held in place as ridigly as in an ionic solid. ...
... delocalized valence electrons. Arises through the shared pooling of valence electrons from many atoms (Electron Sea Model). Leads to crystalline solids Metal ions not held in place as ridigly as in an ionic solid. ...
Atomic Emission Spectra, Electron Configuration, Periodicity
... the alkaline metals, the halogens, the noble gases, the transition metals. Take note that your book refers to these groups as 1A-8A, and 3B-7B. TRANSLATE THESE INTO GROUPS 1-18! 2. Which group numbers correspond to the representative elements, and why are they given that name? 3. Define atomic radiu ...
... the alkaline metals, the halogens, the noble gases, the transition metals. Take note that your book refers to these groups as 1A-8A, and 3B-7B. TRANSLATE THESE INTO GROUPS 1-18! 2. Which group numbers correspond to the representative elements, and why are they given that name? 3. Define atomic radiu ...
Term paper
... In first case, the electron probability is mostly between the nucleii while in second case, it is outside. So, (+) is more stable. We can extend our calculations to p-orbitals and we find similar wave functions. But, other orbitals have directional character. When, pz has formed σ bond, there is lat ...
... In first case, the electron probability is mostly between the nucleii while in second case, it is outside. So, (+) is more stable. We can extend our calculations to p-orbitals and we find similar wave functions. But, other orbitals have directional character. When, pz has formed σ bond, there is lat ...
WEEK 2: 16 J
... electromagnetic spectrum is this emission found? 4B. Determine the final value of n associated with this emission? (Hint: Consider E = h and the Rydberg equation, use this to find the value of nf.) 4C. Determine the initial value of n associated with this emission. (Hint: Will this value be higher o ...
... electromagnetic spectrum is this emission found? 4B. Determine the final value of n associated with this emission? (Hint: Consider E = h and the Rydberg equation, use this to find the value of nf.) 4C. Determine the initial value of n associated with this emission. (Hint: Will this value be higher o ...
Structure of atoms and solids
... The high electrical and thermal conductivities of metals follows from the ability of these free electrons to freely move throughout their crystal structure. This is not the case in covalent or ionic bonding where electrons are tightly bound to single or groups of atoms. Unlike other crystals, metals ...
... The high electrical and thermal conductivities of metals follows from the ability of these free electrons to freely move throughout their crystal structure. This is not the case in covalent or ionic bonding where electrons are tightly bound to single or groups of atoms. Unlike other crystals, metals ...
Physics 280/Jones Week 02 In-Class Problems Fall 2014 1
... turned back down to zero, how fast will the fastest electrons travel? Given, the mass of an electron: me = 9.11 × 10−31 kg. Solution: Recall that when the current has come to a full stop, all electrons including the ones with maximum kinetic energy are held onto the plate: ...
... turned back down to zero, how fast will the fastest electrons travel? Given, the mass of an electron: me = 9.11 × 10−31 kg. Solution: Recall that when the current has come to a full stop, all electrons including the ones with maximum kinetic energy are held onto the plate: ...
CHAPTER 7 READING GUIDE – IONIC COMPOUNDS AND METALS
... 11. An ________________ is a negatively charged ion. 12. The ________________ force that holds oppositely charged particles together in an ionic compound is referred to as an ____________________ bond. 13. Many ionic compounds are ________________, which means that they contain only two different el ...
... 11. An ________________ is a negatively charged ion. 12. The ________________ force that holds oppositely charged particles together in an ionic compound is referred to as an ____________________ bond. 13. Many ionic compounds are ________________, which means that they contain only two different el ...
Auger electron spectroscopy
.jpg?width=300)
Auger electron spectroscopy (AES; pronounced [oʒe] in French) is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science. Underlying the spectroscopic technique is the Auger effect, as it has come to be called, which is based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal Zeitschrift für Physik in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in x-ray spectroscopy data. Since 1953 however, AES has become a practical and straightforward characterization technique for probing chemical and compositional surface environments and has found applications in metallurgy, gas-phase chemistry, and throughout the microelectronics industry.