2. Fermi Statistics of Electrons and Some Definitions
... with the vectors k, and the components kx , ky , kz have to be interpreted as quantum numbers, up to now noted as index j in (#j , ϕj ): The state of an electron of the Hamilton operator (2.17) is labeled by the quantum number k and the spin s. The wave length λ = 2π/k ...
... with the vectors k, and the components kx , ky , kz have to be interpreted as quantum numbers, up to now noted as index j in (#j , ϕj ): The state of an electron of the Hamilton operator (2.17) is labeled by the quantum number k and the spin s. The wave length λ = 2π/k ...
Chapter 3 notes
... Atomic orbital – cloud shaped regions where electrons are thought to be located S – orbital = Spherical P – orbital = Peanut D – orbital = Double peanut F – orbital = Far too complex (Flower) Nodes – regions where the probability of finding an electron is very low (eg: No electrons) ...
... Atomic orbital – cloud shaped regions where electrons are thought to be located S – orbital = Spherical P – orbital = Peanut D – orbital = Double peanut F – orbital = Far too complex (Flower) Nodes – regions where the probability of finding an electron is very low (eg: No electrons) ...
Atoms in Combination: The Chemical Bond
... The periodic table of the elements. The weights of the elements increase from left to right. Each vertical column groups elements with similar chemical properties. ...
... The periodic table of the elements. The weights of the elements increase from left to right. Each vertical column groups elements with similar chemical properties. ...
The principles of transmission electron microscopy image formation
... phase of an electron wave in given point and time. The square of EWF represents the intensity of electron beam. The second element is an atomic form factor (AFF), which described interaction of electron with a single atom A. The AFF gives amplitude of electron wave emitted (scattered) at the angle θ ...
... phase of an electron wave in given point and time. The square of EWF represents the intensity of electron beam. The second element is an atomic form factor (AFF), which described interaction of electron with a single atom A. The AFF gives amplitude of electron wave emitted (scattered) at the angle θ ...
Advanced electronic bonding and how these affect molecular shapes
... levels around the atom. • These energy levels are called shells. • Electrons jump to higher energy levels when provided with energy, but will automatically drop back down to the lowest energy level possible. • These energy levels are named 1, 2, 3, 4, 5, 6, 7, 8 and so on. (So far the heaviest eleme ...
... levels around the atom. • These energy levels are called shells. • Electrons jump to higher energy levels when provided with energy, but will automatically drop back down to the lowest energy level possible. • These energy levels are named 1, 2, 3, 4, 5, 6, 7, 8 and so on. (So far the heaviest eleme ...
Lesson 3 Atomic spectra and the Bohr model
... But it is only one mathematical model of the atom. Other more elegant mathematical models exist that don’t refer to waves, .but physicists like using the wave model because they are familiar with waves and their equations. We stick with what we are familiar! ...
... But it is only one mathematical model of the atom. Other more elegant mathematical models exist that don’t refer to waves, .but physicists like using the wave model because they are familiar with waves and their equations. We stick with what we are familiar! ...
Oops !Power Point File of Physics 2D lecture for Today should have
... 1. n-electron system is stable when its total energy is minimum 2.Only one electron can exist in a particular quantum state in an atom...not 2 or more ! 3. Shells & SubShells In Atomic Structure : (a) ignore inter-electron repulsion (crude approx.) (b) think of each electron in a constant "effective ...
... 1. n-electron system is stable when its total energy is minimum 2.Only one electron can exist in a particular quantum state in an atom...not 2 or more ! 3. Shells & SubShells In Atomic Structure : (a) ignore inter-electron repulsion (crude approx.) (b) think of each electron in a constant "effective ...
2.4 Revision 1: There were two atoms. One got hit by an extremely
... molecules; hydrogen chloride, calcium, sodium chloride, ammonia, graphite. h. The reason that metals can conduct electricity, heat and can be bent. Little Willie was a chemist. Little Willie is no more. For what he thought was H2O --- ----Answers A B ...
... molecules; hydrogen chloride, calcium, sodium chloride, ammonia, graphite. h. The reason that metals can conduct electricity, heat and can be bent. Little Willie was a chemist. Little Willie is no more. For what he thought was H2O --- ----Answers A B ...
lewis dot structures
... In the Kernal, or Noble gas, format for designating electron config we write the electron configuration of the atom by: 1) Writing the symbol of the PRECEDING noble gas element in brackets 1) After skipping the electron configuration portion common to both the nobel gas and the atom in question writ ...
... In the Kernal, or Noble gas, format for designating electron config we write the electron configuration of the atom by: 1) Writing the symbol of the PRECEDING noble gas element in brackets 1) After skipping the electron configuration portion common to both the nobel gas and the atom in question writ ...
Chem Final Study Guide Energy How much heat energy must be
... a) Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, Polonium, Astatine. Metalloids share properties with both metals and nonmetals. 50) Looking at the periodic table, list 4 atomic numbers that represent elements with similar chemical properties. Why did you choose those numbers? a) Any 4 nu ...
... a) Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, Polonium, Astatine. Metalloids share properties with both metals and nonmetals. 50) Looking at the periodic table, list 4 atomic numbers that represent elements with similar chemical properties. Why did you choose those numbers? a) Any 4 nu ...
quantum numbers - misshoughton.net
... these diagrams indicate which orbital energy levels are occupied by electrons for an atom or ion In fig.2 on p. 187, as atoms become larger & the main energy levels come closer, some sublevels may overlap Generally the sublevels for a particular value of n, increase in energy in the order of s ...
... these diagrams indicate which orbital energy levels are occupied by electrons for an atom or ion In fig.2 on p. 187, as atoms become larger & the main energy levels come closer, some sublevels may overlap Generally the sublevels for a particular value of n, increase in energy in the order of s ...
Electron Configurations and Periodicity
... the reason this is so important and why we’re dwelling on this so much is that in knowing where the electrons are, we’re going to be able to predict and understand how the atoms come together to form molecules. So, going from hydrogen to helium, what’s different? Well, we know that in the case of he ...
... the reason this is so important and why we’re dwelling on this so much is that in knowing where the electrons are, we’re going to be able to predict and understand how the atoms come together to form molecules. So, going from hydrogen to helium, what’s different? Well, we know that in the case of he ...
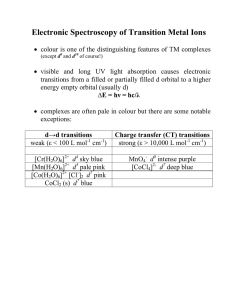
Electronic Spectroscopy of Transition Metal Ions
... With most TM ions, the spin-orbit coupling is small due to electron delocalization onto the ligands so the energy differences between the possible J states are negligible but the possibilities would be J = 4, 3 or 2 giving rise to 3F4, 3F3 and 3F2 states. ...
... With most TM ions, the spin-orbit coupling is small due to electron delocalization onto the ligands so the energy differences between the possible J states are negligible but the possibilities would be J = 4, 3 or 2 giving rise to 3F4, 3F3 and 3F2 states. ...
Electrons in a Shell - University of California, Berkeley
... the boundary (e is the electron charge). The functions ϕ(r) and n(r) can be obtained by numerical integration of the T-F equation [2]. Instead of this, in what follows we prove that for sufficiently large N, the electron density is concentrated in a thin layer of characteristic thickness δ near the ...
... the boundary (e is the electron charge). The functions ϕ(r) and n(r) can be obtained by numerical integration of the T-F equation [2]. Instead of this, in what follows we prove that for sufficiently large N, the electron density is concentrated in a thin layer of characteristic thickness δ near the ...
chapter 7: atomic structure and periodicity
... Quantum numbers describe the various properties of ______________________________. 1. Principal Quantum Number – symbol – has integral values 1,2,3,…..∞ The principal quantum number is related to the _________________ and ____________________ of the orbital. As n increases, the orbital becomes _____ ...
... Quantum numbers describe the various properties of ______________________________. 1. Principal Quantum Number – symbol – has integral values 1,2,3,…..∞ The principal quantum number is related to the _________________ and ____________________ of the orbital. As n increases, the orbital becomes _____ ...
Unit 5 Notes - Har
... These show the actual placement of electrons in individual orbitals. Each orbital is represented as a box, and only orbitals after the last filled noble gas are shown. To do these diagrams, you need to know one more principle. Hund’s rule states that when electrons are being placed in a set of orbit ...
... These show the actual placement of electrons in individual orbitals. Each orbital is represented as a box, and only orbitals after the last filled noble gas are shown. To do these diagrams, you need to know one more principle. Hund’s rule states that when electrons are being placed in a set of orbit ...
Ch 5 Electron ppt
... Chapter 5 focuses on the electrons within atoms for two reasons: 1. Electrons account for the element’s reactivity or “personality” 2. Electrons are the first things that surrounding atoms encounter when they come upon the atom ...
... Chapter 5 focuses on the electrons within atoms for two reasons: 1. Electrons account for the element’s reactivity or “personality” 2. Electrons are the first things that surrounding atoms encounter when they come upon the atom ...
Lewis
... •atomic orbitals and quantum states (with quantum numbers n, m, l, s), • the energy levels of the different shells, subshells, orbitals, • the maximum number of shells, subshells, orbitals, and electrons, • the numbers and/or maximum numbers of bonds, an atom likes to or can build or have, • the Lew ...
... •atomic orbitals and quantum states (with quantum numbers n, m, l, s), • the energy levels of the different shells, subshells, orbitals, • the maximum number of shells, subshells, orbitals, and electrons, • the numbers and/or maximum numbers of bonds, an atom likes to or can build or have, • the Lew ...
Chapter 7(Hill/Petrucci/McCreary/Perry Introduction to Atomic
... Line Spectra and Energy Changes: The H-Atom Label energy levels as integers, where n = 1, 2, 3, … Energy of a given energy level: En = -B/n2 , where B = 2.179 x 10-18 J Transition energy is difference between various energy levels : ∆E = Efinal – Einitial = - B (1/n2 final – 1/n2 iniital) See Exerci ...
... Line Spectra and Energy Changes: The H-Atom Label energy levels as integers, where n = 1, 2, 3, … Energy of a given energy level: En = -B/n2 , where B = 2.179 x 10-18 J Transition energy is difference between various energy levels : ∆E = Efinal – Einitial = - B (1/n2 final – 1/n2 iniital) See Exerci ...
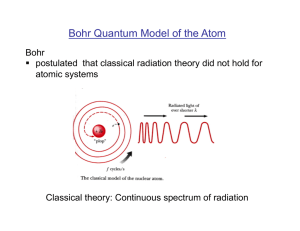
Early Quantum Theory and Models of the Atom
... • Bohr argued that the electrons in a atom also couldn’t lose energy continuously, but must do so in quantum “jumps” • Bohr assumed that the electrons move about in a certain circular orbit • Each orbit have a specific amount of energy • The electrons could move about in that orbit without radiatin ...
... • Bohr argued that the electrons in a atom also couldn’t lose energy continuously, but must do so in quantum “jumps” • Bohr assumed that the electrons move about in a certain circular orbit • Each orbit have a specific amount of energy • The electrons could move about in that orbit without radiatin ...
Auger electron spectroscopy
.jpg?width=300)
Auger electron spectroscopy (AES; pronounced [oʒe] in French) is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science. Underlying the spectroscopic technique is the Auger effect, as it has come to be called, which is based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal Zeitschrift für Physik in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in x-ray spectroscopy data. Since 1953 however, AES has become a practical and straightforward characterization technique for probing chemical and compositional surface environments and has found applications in metallurgy, gas-phase chemistry, and throughout the microelectronics industry.