Lecture28
... No two electrons in an atom can ever have the same set of values for the set of quantum numbers n, l, ml, and ms. • The Pauli exclusion principle explains the electronic structure of complex atoms as a succession of filled levels with different quantum numbers increasing in energy, where the outermo ...
... No two electrons in an atom can ever have the same set of values for the set of quantum numbers n, l, ml, and ms. • The Pauli exclusion principle explains the electronic structure of complex atoms as a succession of filled levels with different quantum numbers increasing in energy, where the outermo ...
Science Starter Tuesday Week 2
... 1,221°F. It turns into a gas at 4566 °F. 1. Can IRON be a liquid? 2. How do I make liquid IRON? 3. Can IRON be a gas? 4. How do I make IRON a gas? 5. T/F. ALL atoms can be a solid, liquid or gas. ...
... 1,221°F. It turns into a gas at 4566 °F. 1. Can IRON be a liquid? 2. How do I make liquid IRON? 3. Can IRON be a gas? 4. How do I make IRON a gas? 5. T/F. ALL atoms can be a solid, liquid or gas. ...
Exercised Review for Test
... 15. The nonmetals in Groups 5A, 6A, and 7A a. lose electrons when they form ions. b. form positively charged ions. c. form ions with charges of 3–, 2–, and 1–, respectively. d. form ions with a numerical charge equal to their group number. 16. Among the following, which atom is most likely to form a ...
... 15. The nonmetals in Groups 5A, 6A, and 7A a. lose electrons when they form ions. b. form positively charged ions. c. form ions with charges of 3–, 2–, and 1–, respectively. d. form ions with a numerical charge equal to their group number. 16. Among the following, which atom is most likely to form a ...
chapter5
... He proposed a planetary model of the atom with the electrons orbiting around the nucleus in a specific circular paths. Each electron has an energy level. Each energy level of the electron can be thought of as rungs on a ladder. The energy levels closest to the nucleus are like rungs of a ladder clos ...
... He proposed a planetary model of the atom with the electrons orbiting around the nucleus in a specific circular paths. Each electron has an energy level. Each energy level of the electron can be thought of as rungs on a ladder. The energy levels closest to the nucleus are like rungs of a ladder clos ...
Chapter 12: Basic Review Worksheet
... 2. What do we mean by ionic bonding? Give an example of a substance whose particles are held together by ionic bonding. 3. What do we mean by covalent bonding and polar covalent bonding? How are these two bonding types similar, and how do they differ? 4. Define electronegativity. 5. What does it mea ...
... 2. What do we mean by ionic bonding? Give an example of a substance whose particles are held together by ionic bonding. 3. What do we mean by covalent bonding and polar covalent bonding? How are these two bonding types similar, and how do they differ? 4. Define electronegativity. 5. What does it mea ...
Electron gun - Wikipedia, the free encyclopedia
... will impinge upon either a red, green or blue phosphor to light up a color pixel on the screen. The resultant http://en.wikipedia.org/wiki/Electron_gun ...
... will impinge upon either a red, green or blue phosphor to light up a color pixel on the screen. The resultant http://en.wikipedia.org/wiki/Electron_gun ...
Ionic and Covalent Bonding
... • the electrons in the highest occupied energy level of an element’s atom ...
... • the electrons in the highest occupied energy level of an element’s atom ...
atomic theory - unit a
... 1) n = principal quantum number, where n is energy shell. Values for n = 1,2,3,4... (n = 1 closest shell to nucleus) Generally, energy increases with increasing n. 2) Principle energy levels can be subdivided into subshells. • Electrons within a subshell have identical energy. • There are 4 known su ...
... 1) n = principal quantum number, where n is energy shell. Values for n = 1,2,3,4... (n = 1 closest shell to nucleus) Generally, energy increases with increasing n. 2) Principle energy levels can be subdivided into subshells. • Electrons within a subshell have identical energy. • There are 4 known su ...
Chapter 9: Atoms
... had no dependence on φ and θ…no for these there are NO bumps in the wavefunction “around” the atom. ...
... had no dependence on φ and θ…no for these there are NO bumps in the wavefunction “around” the atom. ...
nuc_alchemy_talk-fgs-dec07
... atomic ‘vacancies’ i.e. holes in the electron shells around the atom. Quantum mechanics means that the electron orbits are fixed in energy…. X-rays come from an electron ‘dropping’ from one energy level to a lower one ...
... atomic ‘vacancies’ i.e. holes in the electron shells around the atom. Quantum mechanics means that the electron orbits are fixed in energy…. X-rays come from an electron ‘dropping’ from one energy level to a lower one ...
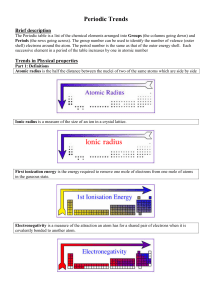
Topic 3 Periodicity notes SL - Chemical Minds
... Going down a group, the atomic radius and ionic radius increase due to an increase in the number of electron shells surrounding the nucleus. The ionisation energy and electronegativity decrease because i) there is a decrease in the electrostatic attraction between the positive protons in the nucleus ...
... Going down a group, the atomic radius and ionic radius increase due to an increase in the number of electron shells surrounding the nucleus. The ionisation energy and electronegativity decrease because i) there is a decrease in the electrostatic attraction between the positive protons in the nucleus ...
Chapter 3 Atomic Structure
... When a flame or other source of energy is absorbed by the electrons, they are promoted to a higher energy state (excited state). When an electron in an excited state returns to a lower energy state, it emits a photon of energy, which may be observed as light. ...
... When a flame or other source of energy is absorbed by the electrons, they are promoted to a higher energy state (excited state). When an electron in an excited state returns to a lower energy state, it emits a photon of energy, which may be observed as light. ...
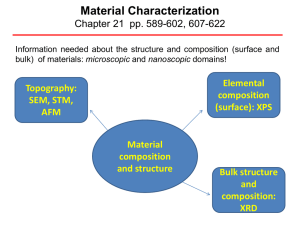
X-ray diffraction techniques X
... gives information on the symmetry of the surface structure. In the presence of an adsorbate the qualitative analysis may reveal information about the size and rotational alignment of the adsorbate unit cell with respect to the substrate unit cell. 2. Quantitatively, where the intensities of diffract ...
... gives information on the symmetry of the surface structure. In the presence of an adsorbate the qualitative analysis may reveal information about the size and rotational alignment of the adsorbate unit cell with respect to the substrate unit cell. 2. Quantitatively, where the intensities of diffract ...
Chapter 6. Electronic Structure of Atoms.
... In order to avoid writing out all the electrons in an atom, the electronic configuration is abbreviated by writing only the electrons in the outermost occupied shell, the valence shell. This is called a condensed electron configuration. e.g. Na: Li: P: ...
... In order to avoid writing out all the electrons in an atom, the electronic configuration is abbreviated by writing only the electrons in the outermost occupied shell, the valence shell. This is called a condensed electron configuration. e.g. Na: Li: P: ...
QuantumDots
... – Higher saturated electron velocity and higher electron mobility than silicon – Gallium arsenide can emit and absorb light, unlike silicon • No silicon laser is possible (or has been made yet) ...
... – Higher saturated electron velocity and higher electron mobility than silicon – Gallium arsenide can emit and absorb light, unlike silicon • No silicon laser is possible (or has been made yet) ...
Solid State Physics II
... electron dynamics E(k), which is obtained from quantum mechanical band structure calculations, determines the electron dynamics It is possible to move between bands but this requires a discontinuous change in the electron’s energy that can be supplied, for example, by the absorption of a photon. ...
... electron dynamics E(k), which is obtained from quantum mechanical band structure calculations, determines the electron dynamics It is possible to move between bands but this requires a discontinuous change in the electron’s energy that can be supplied, for example, by the absorption of a photon. ...
Auger electron spectroscopy
.jpg?width=300)
Auger electron spectroscopy (AES; pronounced [oʒe] in French) is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science. Underlying the spectroscopic technique is the Auger effect, as it has come to be called, which is based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal Zeitschrift für Physik in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in x-ray spectroscopy data. Since 1953 however, AES has become a practical and straightforward characterization technique for probing chemical and compositional surface environments and has found applications in metallurgy, gas-phase chemistry, and throughout the microelectronics industry.