Photoelectric effect
... Figure 1: (a) The potential energy of an electron U = −eφ, where φ is the electrostatic potential, for a metal with work function W and Fermi energy EF . (b) A similar plot for a second metal with work function W 0 and Fermi energy EF ). (Figure from [9]) Now suppose we join two pieces of different ...
... Figure 1: (a) The potential energy of an electron U = −eφ, where φ is the electrostatic potential, for a metal with work function W and Fermi energy EF . (b) A similar plot for a second metal with work function W 0 and Fermi energy EF ). (Figure from [9]) Now suppose we join two pieces of different ...
Chapter: 12 - Physics365.com
... emitting them. The following are the spectral series of hydrogen atom. (i) Lyman series When the electron jumps from any of the outer orbits to the first orbit, the spectral lines emitted are in the ultraviolet region of the spectrum and they are said to form a series called Lyman series . Here, n1 ...
... emitting them. The following are the spectral series of hydrogen atom. (i) Lyman series When the electron jumps from any of the outer orbits to the first orbit, the spectral lines emitted are in the ultraviolet region of the spectrum and they are said to form a series called Lyman series . Here, n1 ...
V. Chemical reactions
... b. Which elements have two valence electrons? Column 2 c. Which elements have three valence electrons? Column 13 d. Which elements have four valence electrons? Column 14 e. Which elements have five valence electrons? Column 15 f. Which elements have six valence electrons? Column 16 g. Which elements ...
... b. Which elements have two valence electrons? Column 2 c. Which elements have three valence electrons? Column 13 d. Which elements have four valence electrons? Column 14 e. Which elements have five valence electrons? Column 15 f. Which elements have six valence electrons? Column 16 g. Which elements ...
Experimental evidence for shell model
... Ionisation potentials and atomic radii: o Ionisation potentials of noble gas elements are highest within a particular period of periodic table, while those of the alkali are lowest. o Ionisation potential gradually increases until shell is filled and then drops. o Filled shells are most stable and v ...
... Ionisation potentials and atomic radii: o Ionisation potentials of noble gas elements are highest within a particular period of periodic table, while those of the alkali are lowest. o Ionisation potential gradually increases until shell is filled and then drops. o Filled shells are most stable and v ...
UV-Vis (electronic) spectroscopy
... • Spin-forbidden transitions – Transitions involving a change in the spin state of the molecule are forbidden – Strongly obeyed – Relaxed by effects that make spin a poor quantum number (heavy atoms) • Symmetry-forbidden transitions – Transitions between states of the same parity are forbidden – Pa ...
... • Spin-forbidden transitions – Transitions involving a change in the spin state of the molecule are forbidden – Strongly obeyed – Relaxed by effects that make spin a poor quantum number (heavy atoms) • Symmetry-forbidden transitions – Transitions between states of the same parity are forbidden – Pa ...
File
... be lost or gained by an atom. Max Planck explained relationship between a quantum of energy and the frequency of radiation: E=hv E=energy in joules v= frequency is s-1 h= Planck’s constant 6.626 X 10-34 J s ...
... be lost or gained by an atom. Max Planck explained relationship between a quantum of energy and the frequency of radiation: E=hv E=energy in joules v= frequency is s-1 h= Planck’s constant 6.626 X 10-34 J s ...
Chapter 28
... • Schrödinger’s wave equation was subsequently applied to hydrogen and other atomic systems - one of the first great achievements of quantum mechanics • The quantum numbers and the restrictions placed on their values arise directly from the mathematics and not from any assumptions made to make the t ...
... • Schrödinger’s wave equation was subsequently applied to hydrogen and other atomic systems - one of the first great achievements of quantum mechanics • The quantum numbers and the restrictions placed on their values arise directly from the mathematics and not from any assumptions made to make the t ...
Exam 2 Sol/81/F01
... calculate means that the constants in that expression will cancel and that all three states have the same L and S quantum numbers, but different J quantum numbers. This gives us ...
... calculate means that the constants in that expression will cancel and that all three states have the same L and S quantum numbers, but different J quantum numbers. This gives us ...
Electrons and Atoms
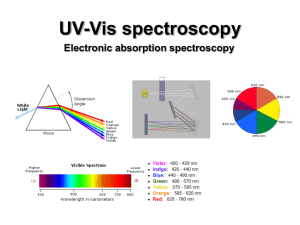
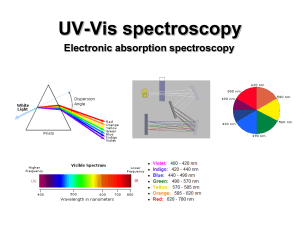
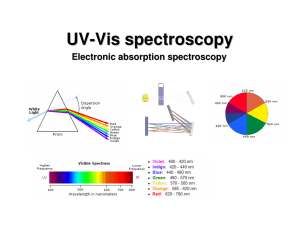
... If the light is not white • By heating a gas with electricity we can get it to give off colors. • Passing this light through a prism does something different. ...
... If the light is not white • By heating a gas with electricity we can get it to give off colors. • Passing this light through a prism does something different. ...
1 - Livonia Public Schools
... Which of the following statements is false? A) An orbital can accommodate at most two electrons. B) The electron density at a point is proportional to psi2 at that point. C) The spin quantum number of an electron must be either +1/2 or –1/2. D) A 2p orbital is more penetrating than a 2s; i.e., it ha ...
... Which of the following statements is false? A) An orbital can accommodate at most two electrons. B) The electron density at a point is proportional to psi2 at that point. C) The spin quantum number of an electron must be either +1/2 or –1/2. D) A 2p orbital is more penetrating than a 2s; i.e., it ha ...
If electrons did not obey the Pauli exclusion Principle then….
... The electrons in an atom would annihilate with the protons in the nucleus The electrons in an atom would all have the same energy The electrons would repel each other preventing the formation of atoms The electrons in an atom would have a continuous range of energies rather than lying in discrete le ...
... The electrons in an atom would annihilate with the protons in the nucleus The electrons in an atom would all have the same energy The electrons would repel each other preventing the formation of atoms The electrons in an atom would have a continuous range of energies rather than lying in discrete le ...
Periodic Properties of the Elements Effective Nuclear Charge, Zeff
... Effective Nuclear Charge, Zeff The splitting of the principle energy level into the s, p, d, and f energy sublevels is best explained by using the concept of “effective” nuclear charge, Zeff. An electron in a higher energy level is “screened” from seeing 100% (all the protons) of the nuclear charge ...
... Effective Nuclear Charge, Zeff The splitting of the principle energy level into the s, p, d, and f energy sublevels is best explained by using the concept of “effective” nuclear charge, Zeff. An electron in a higher energy level is “screened” from seeing 100% (all the protons) of the nuclear charge ...
Chapter 5
... energy level of the electron: 1, 2, 3, etc. • These are called atomic orbitals (coined by scientists in 1932) - regions where there is a high probability of finding an electron. • Sublevels- like theater seats arranged in sections: letters s, p, d, and f ...
... energy level of the electron: 1, 2, 3, etc. • These are called atomic orbitals (coined by scientists in 1932) - regions where there is a high probability of finding an electron. • Sublevels- like theater seats arranged in sections: letters s, p, d, and f ...
Electrons in Atoms
... Principal energy levels can be broken down into sub-levels represented by the letter l. The number of sub-levels in a principal energy level is equal to the principal quantum number. (# of sublevels n = n). There is 1 sub-level in l1, 2 in l2, etc. Sub-levels are generally labeled using the le ...
... Principal energy levels can be broken down into sub-levels represented by the letter l. The number of sub-levels in a principal energy level is equal to the principal quantum number. (# of sublevels n = n). There is 1 sub-level in l1, 2 in l2, etc. Sub-levels are generally labeled using the le ...
Two valence electrons.
... Mendeleev arranged elements by increasing atomic mass, leaving blank spaces where he was sure elements Dmitri yet to be discovered Mendeleev would fit. ...
... Mendeleev arranged elements by increasing atomic mass, leaving blank spaces where he was sure elements Dmitri yet to be discovered Mendeleev would fit. ...
Electron Configuration
... F sublevels The f sublevel is composed of 7 f orbitals. Each orbital is each in the amount of energy. A total of 14 electrons can be found in an f sublevel. ...
... F sublevels The f sublevel is composed of 7 f orbitals. Each orbital is each in the amount of energy. A total of 14 electrons can be found in an f sublevel. ...
Auger electron spectroscopy
.jpg?width=300)
Auger electron spectroscopy (AES; pronounced [oʒe] in French) is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science. Underlying the spectroscopic technique is the Auger effect, as it has come to be called, which is based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal Zeitschrift für Physik in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in x-ray spectroscopy data. Since 1953 however, AES has become a practical and straightforward characterization technique for probing chemical and compositional surface environments and has found applications in metallurgy, gas-phase chemistry, and throughout the microelectronics industry.