From Amino Acids to Proteins - in 4 Easy Steps
... • Two of the polar amino acids (glutamic acid and aspartic acid) contain carboxylic acid functional groups and are therefore acidic (negatively charged). • Two of the polar amino acids (lysine and arginine) contain amino functional groups and are therefore basic (positively charged). • These two ...
... • Two of the polar amino acids (glutamic acid and aspartic acid) contain carboxylic acid functional groups and are therefore acidic (negatively charged). • Two of the polar amino acids (lysine and arginine) contain amino functional groups and are therefore basic (positively charged). • These two ...
Fluorescent High-Throughput Conjugation and Deconjugation
... Topaz GFP acceptor (green) are shown above. When the donor and acceptor are in proximity, FRET is detected. Because of the long fluorescent lifetime of the Tb chelate, FRET can be measured after interfering signals have completely decayed. This, in addition to the ratiometric readout, makes the Lant ...
... Topaz GFP acceptor (green) are shown above. When the donor and acceptor are in proximity, FRET is detected. Because of the long fluorescent lifetime of the Tb chelate, FRET can be measured after interfering signals have completely decayed. This, in addition to the ratiometric readout, makes the Lant ...
PS 1 answers
... Where in a eukaryotic cell do you think you would find the following proteins residing? Be as specific as you can in terms of subcellular location. (a) an enzyme whose substrate is DNA The nucleus. DNA is found in the nucleus of eukaryotic cells, so an enzyme that acts on DNA would have to be found ...
... Where in a eukaryotic cell do you think you would find the following proteins residing? Be as specific as you can in terms of subcellular location. (a) an enzyme whose substrate is DNA The nucleus. DNA is found in the nucleus of eukaryotic cells, so an enzyme that acts on DNA would have to be found ...
comprehensive biochemistry
... (i) Mechanism of CO 2 incorporation, 26 - (i/) ATP production in photoautotrophs; photosystems I and II, 27 g. Biosynthesis of tetrapyrroles h. Gram character and cell-hull structure (i) Cell-wall peptidoglycans, 32 - (ii) The peptides, 33 - (Hi) Bacterial phosphatides, 36 i. Pathways of more genera ...
... (i) Mechanism of CO 2 incorporation, 26 - (i/) ATP production in photoautotrophs; photosystems I and II, 27 g. Biosynthesis of tetrapyrroles h. Gram character and cell-hull structure (i) Cell-wall peptidoglycans, 32 - (ii) The peptides, 33 - (Hi) Bacterial phosphatides, 36 i. Pathways of more genera ...
Protein in disease
... with any post-translational modifications, specify the primary structure of the protein, the fixed chemical bonds. ...
... with any post-translational modifications, specify the primary structure of the protein, the fixed chemical bonds. ...
Chem 400 Biochemistry I
... Temperature - most stabile at low temperature - reduces energy in the system for unfolding and reduces the protease kinetics. Few proteins are unstable at low temps - ppdk (Dr. Chastain's enzyme) and the ATPase in mitochondria Protease inhibitors - several classes of proteins catalyze the hydrol ...
... Temperature - most stabile at low temperature - reduces energy in the system for unfolding and reduces the protease kinetics. Few proteins are unstable at low temps - ppdk (Dr. Chastain's enzyme) and the ATPase in mitochondria Protease inhibitors - several classes of proteins catalyze the hydrol ...
File
... chains of linked simple sugars. Polysaccharides are large insoluble molecules that are ideal storage products. Carbohydrates provide a ready easily usable source of food energy for cells. Polysaccharides are long polymers consisting of up to hundreds of glucose molecules. ...
... chains of linked simple sugars. Polysaccharides are large insoluble molecules that are ideal storage products. Carbohydrates provide a ready easily usable source of food energy for cells. Polysaccharides are long polymers consisting of up to hundreds of glucose molecules. ...
Chapter 1
... first approach has the advantage of being less dependent on the availability of information on function of other proteins. However, it is significantly more expensive computationally and in has a lower success rate when the alternative approach works. The second method, relying on evolutionary links ...
... first approach has the advantage of being less dependent on the availability of information on function of other proteins. However, it is significantly more expensive computationally and in has a lower success rate when the alternative approach works. The second method, relying on evolutionary links ...
PROTEIN TURNOVER AND NITROGEN ECONOMY - U
... - proteins metabolism has a balance between body’s energy and synthetic needs - dietary protein required to synthesize endogenous proteins (albumin, myosin, actin) - essential amino acids cannot be synthesize by body; others can be synthesized from carbon sources -table - protein balance relations ...
... - proteins metabolism has a balance between body’s energy and synthetic needs - dietary protein required to synthesize endogenous proteins (albumin, myosin, actin) - essential amino acids cannot be synthesize by body; others can be synthesized from carbon sources -table - protein balance relations ...
Protein Sequencing
... If first few N-terminal amino acid of a protein is known, complete aminoacid sequence can be derived using Molecular Biology techniques. A simple example is as follow: The genome sequence of Calotropis procera, a plant, or the sequence of procerain B, a novel cystein protease from the plant, gene is ...
... If first few N-terminal amino acid of a protein is known, complete aminoacid sequence can be derived using Molecular Biology techniques. A simple example is as follow: The genome sequence of Calotropis procera, a plant, or the sequence of procerain B, a novel cystein protease from the plant, gene is ...
organic molecules
... the other end, and H and R groups a. portion that differs: R-group 2. More than 20 different amino acids in nature 3. Sequence of amino acids determines the protein C. 2 amino acids joined by a peptide bond forms a dipeptide. A long chain is called a polypeptide. D. Proteins help control the rate of ...
... the other end, and H and R groups a. portion that differs: R-group 2. More than 20 different amino acids in nature 3. Sequence of amino acids determines the protein C. 2 amino acids joined by a peptide bond forms a dipeptide. A long chain is called a polypeptide. D. Proteins help control the rate of ...
Peptides and Protein Primary Structure
... provides recognition (binding) sites for interaction with other proteins Fatty acylation of α-amino group (amide linkage) or of specific cysteine residues (thioester linkage) makes protein more hydrophobic sometimes helps tether protein to membrane ADP-ribosylation (addition of an ADP-ribosyl group) ...
... provides recognition (binding) sites for interaction with other proteins Fatty acylation of α-amino group (amide linkage) or of specific cysteine residues (thioester linkage) makes protein more hydrophobic sometimes helps tether protein to membrane ADP-ribosylation (addition of an ADP-ribosyl group) ...
NMEICT PROJECT
... 17. When a colored solution absorbs light maximally at a particular wavelength then that wavelength is known as ________________ wavelength. 3. All carbohydrates contain carbon, hydrogen and oxygen. All proteins contain carbon, hydrogen, oxygen and what other element ...
... 17. When a colored solution absorbs light maximally at a particular wavelength then that wavelength is known as ________________ wavelength. 3. All carbohydrates contain carbon, hydrogen and oxygen. All proteins contain carbon, hydrogen, oxygen and what other element ...
19-7-SA-V1-S1__mcq_a..
... 17. When a colored solution absorbs light maximally at a particular wavelength then that wavelength is known as ________________ wavelength. 3. All carbohydrates contain carbon, hydrogen and oxygen. All proteins contain carbon, hydrogen, oxygen and what other element ...
... 17. When a colored solution absorbs light maximally at a particular wavelength then that wavelength is known as ________________ wavelength. 3. All carbohydrates contain carbon, hydrogen and oxygen. All proteins contain carbon, hydrogen, oxygen and what other element ...
Large-scale Protein Flexibility Analysis of Single Nucleotide
... Amino acids (aa): Building blocks for proteins, 20 different aa are ...
... Amino acids (aa): Building blocks for proteins, 20 different aa are ...
Mini-Review Roles of Molecular Chaperones in Protein Degradation
... EAT and other forms of stress that cause proteins to denature induce the synthesis of several classes of proteins known as heat shock proteins ( h s p s ) 1 many of which act as molecular chaperones (see Table I). A major role of these molecular chaperones after stress is to catalyze the refolding o ...
... EAT and other forms of stress that cause proteins to denature induce the synthesis of several classes of proteins known as heat shock proteins ( h s p s ) 1 many of which act as molecular chaperones (see Table I). A major role of these molecular chaperones after stress is to catalyze the refolding o ...
Additional Lab Exercise: Amino Acid Sequence in
... Background Information Enzymes are proteins. In order to carry on their very specific functions, the sequence of the amino acids in their structure must be precise. The DNA in the chromosomes of cells, through its own order of bases, is the determining factor in the amino acid sequence. Ribosomes, m ...
... Background Information Enzymes are proteins. In order to carry on their very specific functions, the sequence of the amino acids in their structure must be precise. The DNA in the chromosomes of cells, through its own order of bases, is the determining factor in the amino acid sequence. Ribosomes, m ...
Lecture 5: Major Nutrient Groups
... primary: the sequence of AA’s forming the protein secondary: forces generated by the close proximity of one AA residue to another (e.g., helix design or pleated sheet)(i.e., certain amino acids can form bonds ...
... primary: the sequence of AA’s forming the protein secondary: forces generated by the close proximity of one AA residue to another (e.g., helix design or pleated sheet)(i.e., certain amino acids can form bonds ...
Cell Standards
... forms and functions. For example, all organisms require an outside source of energy to sustain life processes; all organisms demonstrate patterns of growth and, in many cases, senescence, the process of becoming old; and the continuity of all species requires reproduction. All organisms are construc ...
... forms and functions. For example, all organisms require an outside source of energy to sustain life processes; all organisms demonstrate patterns of growth and, in many cases, senescence, the process of becoming old; and the continuity of all species requires reproduction. All organisms are construc ...
Name: TF Name: 1
... 164. The numbers indicate the amino acid’s position in the protein’s primary sequence (where “1” is the amino acid at the N-terminus and “372” is the amino acid at the C-terminus of this particular protein). Based on these numbers, fill in the blank next to each of the two indicated amino acids to s ...
... 164. The numbers indicate the amino acid’s position in the protein’s primary sequence (where “1” is the amino acid at the N-terminus and “372” is the amino acid at the C-terminus of this particular protein). Based on these numbers, fill in the blank next to each of the two indicated amino acids to s ...
Protein Synthesis: Translation
... 3) A transfer RNA with an amino acid is called a charged tRNA. (An enzyme and ATP bind to the correct amino acid to the transfer RNA molecule. At that point it is ready to carry the amino acid to its correct place in the growing polypeptide chain.) ...
... 3) A transfer RNA with an amino acid is called a charged tRNA. (An enzyme and ATP bind to the correct amino acid to the transfer RNA molecule. At that point it is ready to carry the amino acid to its correct place in the growing polypeptide chain.) ...
New study illuminates ability of hot
... genetic information contained in the cell's DNA. This information is transferred via molecules known as messenger RNA, in a process called translation. The team was able to identify the exact part of the messenger RNA helix that the RbfA protein acts on during protein construction - it acts to ensur ...
... genetic information contained in the cell's DNA. This information is transferred via molecules known as messenger RNA, in a process called translation. The team was able to identify the exact part of the messenger RNA helix that the RbfA protein acts on during protein construction - it acts to ensur ...
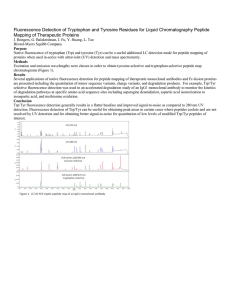
Fluorescence Detection of Tryptophan and Tyrosine Residues for
... Native fluorescence of tryptophan (Trp) and tyrosine (Tyr) can be a useful additional LC detection mode for peptide mapping of proteins when used in-series with ultraviolet (UV) detection and mass spectrometry. Methods Excitation and emission wavelengths were chosen in order to obtain tyrosine-selec ...
... Native fluorescence of tryptophan (Trp) and tyrosine (Tyr) can be a useful additional LC detection mode for peptide mapping of proteins when used in-series with ultraviolet (UV) detection and mass spectrometry. Methods Excitation and emission wavelengths were chosen in order to obtain tyrosine-selec ...
Proteolysis
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion. Low pH or high temperatures can also cause proteolysis non-enzymatically.Proteolysis in organisms serves many purposes; for example, digestive enzymes break down proteins in food to provide amino acids for the organism, while proteolytic processing of a polypeptide chain after its synthesis may be necessary for the production of an active protein. It is also important in the regulation of some physiological and cellular processes, as well as preventing the accumulation of unwanted or abnormal proteins in cells. Consequently, dis-regulation of proteolysis can cause diseases, and is used in some venoms to damage their prey.Proteolysis is important as an analytical tool for studying proteins in the laboratory, as well as industrially, for example in food processing and stain removal.